Figure 2.
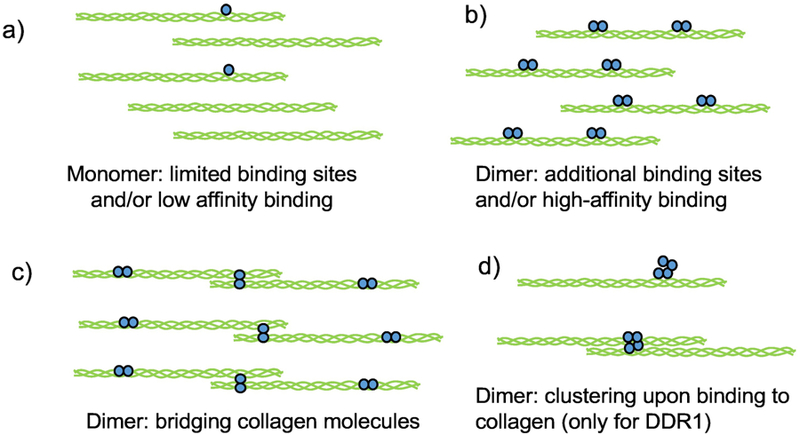
Possible mechanisms explaining enhanced binding of dimeric (or oligomeric DDR ECDs (shown in blue) to collagen (shown in green). As compared to monomers (a) dimers of DDR ECD (b) may bind to a high-affinity binding site as well as an adjacent low-affinity site thus enhancing the overall affinity. This could lead to reduced dissociation of the bound DDR ECD thus increasing the number of binding events on collagen molecules present. In addition dimers may bind to additional sites on the collagen triple helix (b) or to an end-to-end overlap of collagen molecules (c), which may not happen with DDR monomers. Clustering of DDR1 ECD can also increase the amount of bound protein present on collagen (d). While dimeric DDR1 ECD has been shown to cluster, clustering of monomeric DDR1 is not known.
