Figure 3.
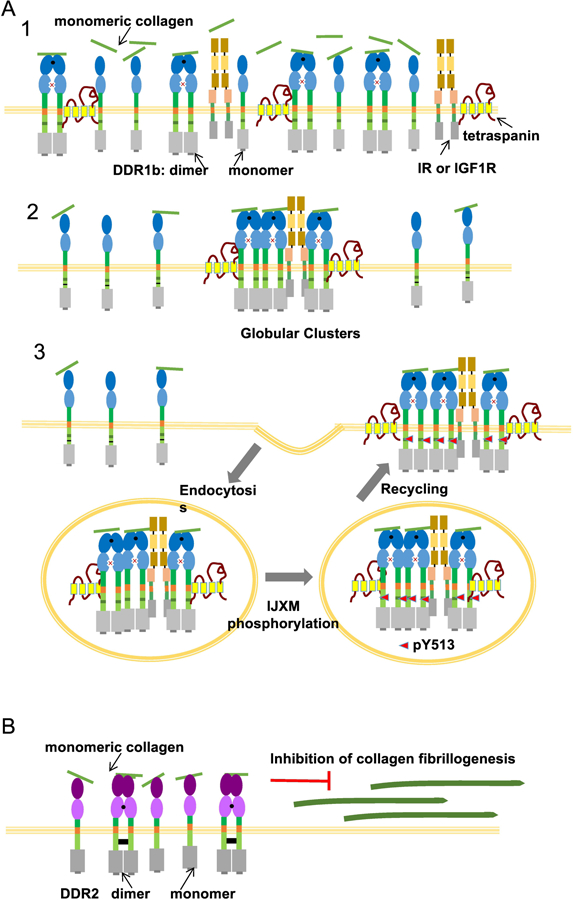
(a): Clustering of DDR1b in response to monomeric collagen. Upon exposure to soluble, monomeric collagen, high-affinity DDR1b dimers bind to collagen (as in 1) and rapidly undergo clustering (as in 2). Thus, while clustering of DDR1b sequesters the dimer population, it remains to be examined if monomers are also incorporated in clusters. DDR1b clusters also recruit other membrane components like the insulin receptor (IR), insulin-like growth factor receptor (IGF1R) and tetraspanins. The DDR1b clusters thus formed are endocytosed to early endosomes, likely via a clathrin mediated endocytosis pathway. The clusters recycle back to the cell membrane after several hours and exhibit DDR1b phosphorylation at Y513 in its IJXM region (as in 3). Since tyrosine phosphorylation in KD does not occur in these clusters the ensuing signaling due to DDR1b clusters is termed non-canonical signaling. While this process is likely to be present in DDR1c due to NPXY motif in its IJXM as in DDR1b, it remains to be investigated if DDR1a also undergoes clustering and endocytosis. (b) Unlike DDR1b, DDR2 does not undergo clustering or endocytosis in response to monomeric collagen. Binding of high-affinity dimers to monomeric collagen inhibits collagen fibril formation on the cell surface.
