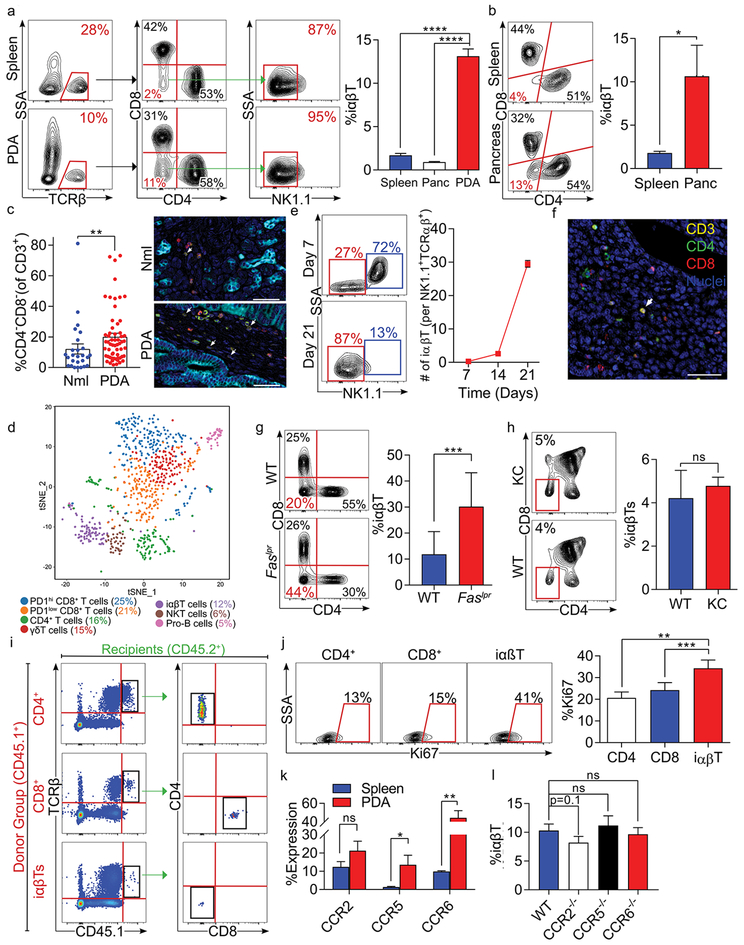
Figure 1. iαβTs expand in PDA.
(a) CD45+ leukocytes infiltrating day 21 orthotopic KPC tumors, normal pancreas, and spleens in WT mice were gated and tested for the frequency of TCRβ+CD4–CD8–NK1.1– iαβTs. Representative contour plots and quantitative data are shown (n=10). (b) CD45+TCRβ+ NK1.1– leukocytes from pancreata and spleens of 6 month-old KC mice were gated and tested for co-expression CD4 and CD8. Representative contour plots are shown (n=5). (c) Multiplex IHC of human PDA and adjacent normal pancreas were stained for CK19, CD3, CD4, and CD8. The frequency of CD3+CD4–CD8– cells were quantified and representative images are shown. (d) Orthotopic KPC tumors were harvested from WT mice on day 21. CD45+CD3+ leukocytes were purified by FACS and analyzed by single cell RNAseq. The distribution of cellular clusters was determined using the t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm. Each cluster is identified by a distinct color. Percent cellular abundance in each cluster is indicated. (e) Orthotopic KPC tumors were harvested from WT mice on days 7, 14, or 21 after tumor cell implantation and tumor-infiltrating CD45+TCRβ+CD4–CD8– leukocytes were gated and tested for expression of NK1.1. Representative contour plots from days 7 and 21 and quantitative data comparing frequency of tumor-infiltrating iαβT per NKT cells at all time points are shown (n=5/time point). (f) Paraffin-embedded sections made from tumors of mice serially treated with anti-TCRγ/δ and NK1.1 depleting antibodies were tested for co-expression of Hematoxylin, CD3, CD4, and CD8 in the PDA TME. (g) CD45+TCRβ+NK1.1– leukocytes infiltrating orthotopic KPC tumors in WT and Faslpr mice were gated and tested for expression of CD4 and CD8. Representative contour plots and quantitative data are shown (n=5/group). (h) The thymus from 6-month old WT and KC mice were harvested and CD45+TCRβ+ thymocytes were gated and tested for expression of CD4 and CD8. The frequency of iαβTs in the thymus was calculated (n=5/group). (i) CD4+ T cells, CD8+ T cells, or iαβTs were harvested from CD45.1 mice and transferred i.v. to orthotopic PDA-bearing CD45.2 mice. PDA tumors were harvested at 96 hours and CD45.1+ cells were gated and tested for CD4 and CD8 expression. Representative contour plots are shown (n=5/group). (j) WT mice were orthotopically administered KPC tumor cells and sacrificed on day 21. PDA-infiltrating CD4+ T cells, CD8+ T cells, and iαβTs were assayed for Ki67 proliferative index. Representative contour plots and quantitative data are shown (n=5). (k) Splenic and orthotopic PDA-infiltrating iαβTs were tested on day 21 for expression of CCR2, CCR5, and CCR6 (n=5). (l) The frequency of PDA-infiltrating iαβTs was tested on day 21 in WT, CCR2–/–, CCR5–/–, and CCR6–/– hosts (n=5/group). All experiments were repeated at least 3 times (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).