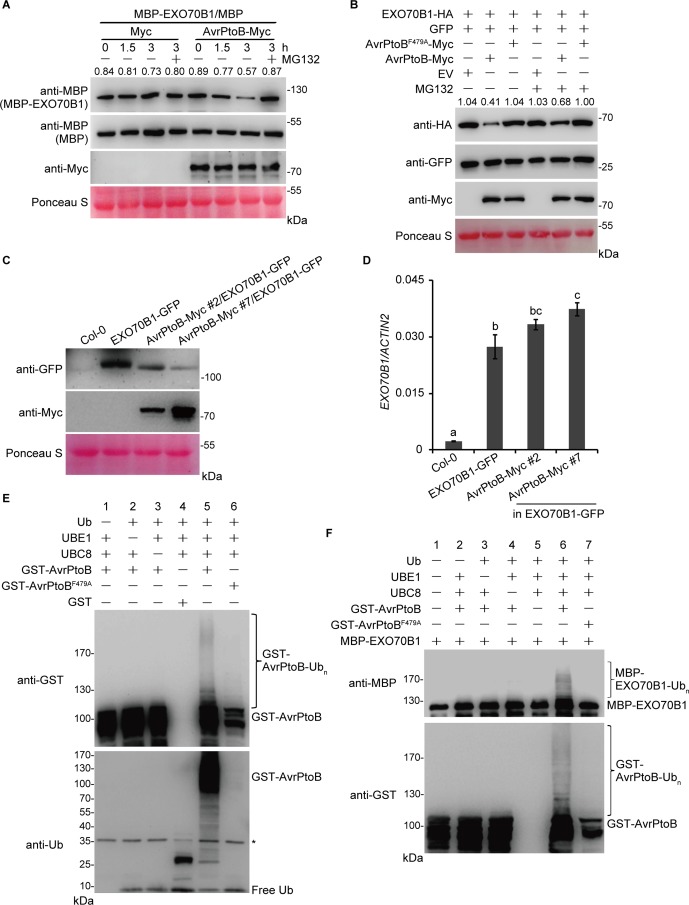
Figure 3.
The degradation and ubiquitination of EXO70B1 by AvrPtoB. (A) Cell-free degradation assay of EXO70B1 by AvrPtoB. Bacterially expressed MBP or MBP-EXO70B1 was incubated with a cell-free crude extract prepared from N. benthamiana leaves transiently expressing AvrPtoB-Myc or Myc, in the presence or absence of 50 μM of MG132. The time-dependent changes of protein levels were monitored by immunoblotting with anti-MBP antibody. Numbers indicate the protein levels of MBP-EXO70B1 normalized to MBP using ImageJ software. The levels of Rubisco are shown as an equal loading control of cell-free extracts. (B) In vivo degradation assay of EXO70B1 by AvrPtoB in protoplasts. The 35S:EXO70B1-HA and 35S:GFP constructs were cotransfected with the 35S:Myc, 35S:AvrPtoB-Myc, or 35S:AvrPtoBF479A-Myc constructs into protoplasts freshly isolated from wild-type Arabidopsis leaf tissues using PEG-mediated transformation. After overnight transfection, protoplasts were incubated with or without 50 μM of MG132 for 3 h. Total proteins were extracted and examined by immunoblot analysis with anti-HA, anti-GFP, and anti-Myc antibodies. The levels of GFP show the transfection efficiency of protoplasts. Numbers indicate the protein levels of EXO70B1-HA normalized to GFP using ImageJ software. The levels of Rubisco are shown as an equal loading control. (C) EXO70B1-GFP and AvrPtoB-Myc levels in the indicated genotypes were determined by immunoblotting using anti-GFP and anti-Myc antibodies. Ponceau S staining of Rubisco is shown as a protein loading control. (D) RT-qPCR analysis of EXO70B1 expression. Total RNA was isolated from leaves of the 5-week-old Arabidopsis plants. The expression levels of EXO70B1 were normalized to the reference gene ACTIN2. Data represent the mean and standard deviation of three biological replicates. Three technical replicates for each biological sample were used. The lowercase letters indicate statistically significant differences (P < 0.05; one-way ANOVA). (E) In vitro self-ubiquitination assay of AvrPtoB. Recombinant GST-AvrPtoB was incubated in ubiquitination buffer in the presence or absence of ubiquitin, E1 (UBE1), or E2 (UBC8) at 30°C for 2 h. Recombinant GST-AvrPtoBF479A was used as a negative control. Reaction products were resolved using SDS-PAGE and subjected to immunoblot analysis with anti-GST and anti-Ub antibodies. Asterisk indicates the contaminating bands. (F) In vitro ubiquitination assay of EXO70B1 by AvrPtoB. Recombinant MBP-EXO70B1 was incubated in ubiquitination buffer in the presence or absence of ubiquitin, E1 (UBE1), E2 (UBC8), or E3 (AvrPtoB or AvrPtoBF479A). The reaction mixtures were subjected to immunoblot analysis with anti-MBP and anti-GST antibodies. Ubiquitinated MBP-EXO70B1 was detected by anti-MBP antibody. These experiments were repeated three times with similar results.