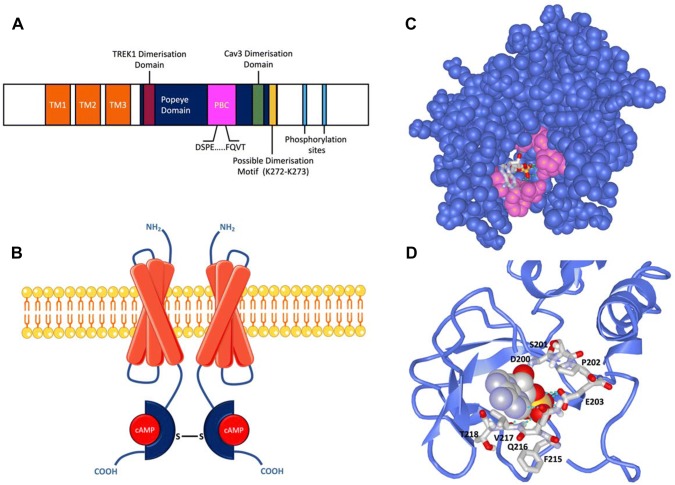
Fig. 2.
Structure and Function of the Popeye domain containing proteins. a Schematic of the general, linear structure of the POPDC proteins. The short amino terminus contains one (POPDC2 or POPDC3) or two (POPDC) N-glycosylation sites. Three transmembrane domains (TM) are followed by the intracellular Popeye domain, which contains the PBC, including the conserved DSPE and FQVT motifs. The putative POPDC dimerization motif (K272/K273) (Kawaguchi et al. 2008), is shown along with the proposed interaction sites of TREK1 (Schindler et al. 2016) and CAV3 (Alcalay et al. 2013). The carboxy terminus contains a number of conserved phosphorylation sites, which are subject to phosphorylation in response to adrenergic stimulation (Lundby et al. 2013). b A schematic of the quaternary structure of a membrane associated POPDC dimer. Each Popeye domain is shown bound to a molecule of cAMP (red sphere). In case of POPDC1 an intramolecular disulphide bridge stabilises the homodimer. c A homology model of the Popeye domain of POPDC1. The conserved DSPE and FQVT motifs, part of the proposed PBC, are shown in pink, with a molecule of cAMP bound. d An enlargement of the cAMP bound PBC from the model shown in c. The position of the DSPE and FQVT motifs are indicated. The models in C and D were created using the Phyre 2 algorithm (Kelley et al. 2015) and the image was rendered using iCn3D (iCn3D 2016)