Fig. 3.
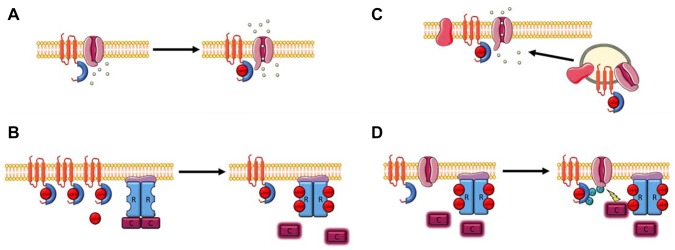
Four proposed working models of the role of POPDC proteins in the cAMP pathway. a The switch model. Binding of cAMP to POPDC leads to a direct change in activity of an interaction partner e.g. opening of TREK1. b The sponge model. The binding of cAMP to POPDC proteins leads to a reduction in the local cAMP concentration. This reduces activation of other proteins in the cAMP pathway, in this case PKA. A lowering of POPDC expression (such as in null mutants), or alternatively a reduction in the cAMP binding affinity (such as in missense mutations found in patients), leads to an increase in the free cAMP concentration and therefore results in stronger and more sustained activation of cAMP effector proteins. c Cargo model. This model proposes that cAMP influences the POPDC protein’s role in modulating membrane influences the POPDC protein’s role in modulating membrane expression of interaction partners. The exact effect of cAMP binding on vesicle transport and membrane trafficking is currently not fully understood. d The shield model. This model builds on the switch model and suggests that cAMP binding to the Popeye domain may lead to indirect downstream effects, possibly via phosphorylation of the POPDC proteins. For example, in the unbound state POPDC proteins may shield interaction partners from being accessed by kinases, with this inhibition being reduced after cAMP binding to the Popeye domain. How the phosphorylation and conformational changes of POPDC proteins after cAMP mediate downstream effects is still unclear