Abstract
Background
Although a large number of studies on heart failure with reduced ejection fraction (HFrEF) have found that anemia and renal dysfunction (RD) independently predicted poor outcomes, there are still few reports on patients with heart failure with preserved ejection fraction (HFpEF).
Methods
Clinical data of HFpEF patients registered in the China National Heart Failure Registration Study (CN-HF) were evaluated and the clinical features of patients with or without anemia/RD were compared to explore the impact of anemia and RD on all-cause mortality and all-cause re-hospitalization.
Results
1604 patients with HFpEF were enrolled, the prevalence of anemia was 51.0%. Although anemia was associated with increased risk of all-cause mortality and all-cause re-hospitalization in univariate COX regression (p < 0.05), multivariate COX model confirmed that anemia was not independently associated with all-cause mortality [hazard ratio (HR) 1.14, 95% confidence interval (CI) 0.85–1.52, p = 0.386] and all-cause re-hospitalization (HR 1.13, 95% CI 0.96–1.33, p = 0.152). Similarly, RD was not an independent predictor of all-cause mortality (HR 1.18, 95% CI 0.88–1.57, p = 0.269) and all-cause re-hospitalization (HR 0.94, 95% CI 0.79–1.12, p = 0.488) as assessed in the adjusted COX regression model. The interaction between RD and anemia on end-points events was also not statistically significant. However, anemia was associated with increased all-cause re-hospitalization in patients with New York Heart Association (NYHA) class III-IV.
Conclusions
In patients with HFpEF from CN-HF registry, anemia was common, but was not an independent predictor of all-cause mortality and all-cause re-hospitalization, except for the all-cause re-hospitalization in patients with NYHA class III-IV.
Clinical Trial Registration: http://www.clinicaltrials.gov/ct2/home; ID: NCT02079428.
Abbreviations and acronyms: HFrEF, heart failure with reduced ejection fraction; RD, renal dysfunction; HFpEF, heart failure with preserved ejection fraction; CN-HF, China National Heart Failure Registration Study; HR, hazard ratio; CI, confidence interval; NYHA, New York Heart Association; HF, Heart failure; CRFs, case report forms; LVEF, left ventricular ejection fraction; LVDD, left ventricular diastolic dimension; AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; TC, serum total cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglyceride; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonist
Keywords: Heart failure, Heart failure with preserved ejection fraction, Anemia, Renal dysfunction
1. Introduction
Heart failure (HF) is the severe and end-stage of many cardiovascular diseases, anemia and renal dysfunction (RD) are common comorbidities of HF [1]. The prevalence of anemia in HF patients is approximately 4%–58%, varies widely in different regions and is associated with poor prognosis [[2], [3], [4], [5]]. A large number of studies on heart failure with reduced ejection fraction (HFrEF) have found that anemia and RD independently predicted a worse outcome, and intravenous iron supplementation can improve cardiac function, quality of life and outcome of HF [5,6].
Heart failure with preserved ejection fraction (HFpEF) has received intensive research attention in recent years, the proportion of HFpEF is as high as 22%–73% in HF patients [5]. It was shown that the prevalence of anemia was similar in patients with HFrEF and HFpEF [7,8]. However, the prognostic information of coexisting anemia and RD in patients with HFpEF is poorly described. In this study, it was sought to determine the association between RD and anemia in patients with HFpEF enrolled in the prospective, multicenter China National Heart Failure Registration Study (CN-HF).
2. Methods
2.1. Study population
We used the data from CN-HF (ID: NCT02079428). CN-HF, sponsored by Ministry of Science and Technology of the People's Republic of China, was a nationwide, hospital-based, multicenter, prospective registry study. This cohort consisted of 7171 HF patients enrolled from 45 hospitals across nine regions in China. Inclusion criteria for CN-HF studies were: (1) Patients discharged from the Department of Cardiology or Cardiovascular; (2) HF was included in the discharge diagnosis or dead diagnosis during hospitalization; (3) Informed consent form was given. Exclusion criteria were: (1) Patients who did not agree to sign an informed consent form; (2) Patients who had participated in the study during the previous hospitalization. Data from the eligible patients were obtained from medical records as well as from face-to-face interviews. All data were recorded in standardized case report forms (CRFs), which were completed before hospital discharge. This recorded information included mainly history and physical examination, diagnostic tests (biomarkers, noninvasive cardiac imaging, invasive evaluation when applicable), treatment (pharmacological treatment, device), in-hospital outcome, follow-up and long-term outcome. Of about 200 variables, 67 variables were mandatory while others were optional.
The study conformed to the Helsinki Declaration and approved by all participating hospital ethics committees.
2.2. Definition of HFpEF
HFpEF was defined by the criteria from Chinese guidelines for the diagnosis and treatment of heart failure 2014 [9], composing the presence of 4 of the following components: (1) Heart failure symptoms and signs; (2) left ventricular ejection fraction (LVEF) ≥50%; (3) Normal-sized left ventricle: left ventricular diastolic dimension (LVDD) <60 mm or < 97 ml/m2; and one of the following echocardiographic parameters: a. Left ventricular hypertrophy: >115 g/m2 for men or > 95 g/m2 for women; b. Left atrial enlargement: >40 mm or > 34 ml/m2; c. Tissue Doppler-verified diastolic dysfunction; (4) Exclusion of valvular heart disease, pericardial disease, hypertrophic cardiomyopathy, or restrictive cardiomyopathy.
2.3. Data collection
Records from all potential HFpEF hospitalizations were obtained from CN-HF main database. The demographic data, comorbidities, physical examination, laboratory indicators, echocardiography, current medication and clinical outcomes were analyzed. Among them, physical examination, laboratory indicators and echocardiography resulted from admission examinations, the comorbidities specifically included the previous medical history and the first diagnosis by the clinician after admission, atrial fibrillation (AF) included a history of previous AF or AF confirmed by electrocardiography after admission.
Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) equations [10], eGFR (ml·min−1·1.73 m−2) = 186 × (creatinine) − 1.154 × (age) − 0.203 × (female × 0.742). RD was defined by an eGFR < 60 ml·min−1·1.73 m−2 at admission, and anemia was defined as hemoglobin (Hb) < 130 g/l in males and <120 g/l in females according to the World Health Organization criteria (WHO) [11].
The CN-HF study cohort patients were followed up for at least 3 years after discharge by telephone or outpatient visits every 3–6 month. In this study, our study sample was 1604 patients, after excluding baseline patients who did not meet the HFpEF diagnosis and died during hospitalization (Fig. 1).
Fig. 1.
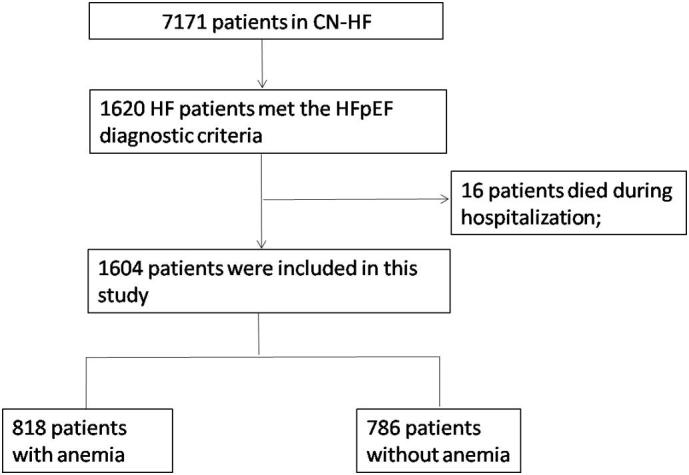
Flowchart. CN-HF = China National Heart Failure Registration Study; HF = heart failure; HFpEF = heart failure with preserved ejection fraction;
2.4. Statistical methods
We described the prevalence of anemia in HFpEF patients, compared the baseline characteristics of patients with anemia and those with non-anemia, and analyzed the impact of anemia and RD on all-cause mortality and all-cause re-hospitalization. Then, the patients were divided into the following four groups: patients with neither anemia nor renal dysfunction (Anemia-RD-), patients with anemia but no renal dysfunction (Anemia+RD−), patients with renal dysfunction but no anemia (Anemia-RD+), patients with both anemia and renal dysfunction (Anemia+RD+) to further analyze the impacts of anemia and renal dysfunction interactions on the outcomes. In addition, we also performed a subgroup analysis of NYHA class.
Summary statistics (means, SDs, median, interquartile range and proportions) were used to characterize the study population as appropriate. Student's t-test or Mann-Whitney U test were used to compare the continuous variables whereas chi-square test or Fisher's exact test were used to compare the categorical date. Kaplan-Meier curves and log-rank test were used to describe time to events and compare the outcomes. Multivariable COX proportional hazard regression model was performed to assess the hazard ratio (HR) of all-cause mortality associated with anemia and RD, and the following covariates were included in the model: baseline age, gender, hypertension, diabetes mellitus, myocardial infarction, hyperlipidemia, atrial fibrillation, heart rate, systolic blood pressure, diastolic blood pressure, NYHA classes, LVEF, eGFR < 60 ml·min−1·1.73 m−2, serum sodium, serum potassium, serum total cholesterol (TC), high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), triglyceride (TG), brain natriuretic peptide (BNP)/N-terminal pro-brain natriuretic peptide (NT-proBNP), use of angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), beta-blockers, digital, mineralocorticoid receptor antagonist (MRA), nitrates, stain, aspirin, traditional Chinese medicine and anticoagulant therapy. Statistical analyses were performed using SPSS 23.0. Two-tailed p < 0.05 were considered to indicate statistical significance.
3. Results
Of the 1604 patients, 818 (51.0%) patients were diagnosed as anemia. Patients with anemia were older, had higher NYHA class and higher levels of serum potassium and NT-proBNP, were more likely to receive MRAs and Nitrates; while fewer smokers and drinkers, lower heart rate and diastolic blood pressure (DBP), lower eGFR, lower serum level of LDL and TG, less comorbidity of hyperlipidemia, and less use of ACEIs/ARBs, stain and aspirin as compared to patients without anemia (Table 1).
Table 1.
Baseline characteristics in HFpEF patients with anemia and without anemia.
| Characteristics | All (n = 1604) | Non-anemia (N = 786) | Anemia (N = 818) | p-Values |
|---|---|---|---|---|
| Age (years) | 74.3 ± 11.3 | 71.6 ± 11.8 | 76.9 ± 10.2 | <0.001 |
| Male | 852(53.1%) | 427(54.3%) | 425(52.0%) | 0.342 |
| Smoking | 410(25.6%) | 227(28.9%) | 184(22.5%) | 0.003 |
| Drinking | 161(10.0%) | 91(11.6) | 70(8.6%) | 0.044 |
| Comorbidities | ||||
| Hypertension | 1184(73.8%) | 573(72.9%) | 611(74.7%) | 0.414 |
| Diabetes mellitus | 439(27.4%) | 198(25.2%) | 241(29.5%) | 0.055 |
| Hyperlipidemia | 102(6.4%) | 60(7.6%) | 42(5.1%) | 0.040 |
| Myocardial infarction | 212(13.2%) | 108(13.7%) | 104(12.7%) | 0.544 |
| Atrial fibrillation | 646(40.3%) | 307(39.1%) | 339(41.4%) | 0.330 |
| Physical examination | ||||
| Heart rate (beats/min) | 79.7 ± 20.0 | 80.8 ± 20.8 | 78.8 ± 18.9 | 0.041 |
| Systolic blood pressure (mmHg) | 138.7 ± 23.8 | 138.3 ± 22.8 | 139.0 ± 24.6 | 0.542 |
| Diastolic blood pressure (mmHg) | 78.6 ± 13.6 | 80.4 ± 13.3 | 76.8 ± 13.7 | <0.001 |
| NYHA classes | <0.001 | |||
| I | 57(3.6%) | 35(4.5%) | 22(2.7%) | |
| II | 699(43.6%) | 372(47.3%) | 327(40.0%) | |
| III | 683(42.6%) | 320(40.7%) | 363(44.4%) | |
| IV | 165(10.3%) | 59(7.5%) | 106(13.0%) | |
| LVEF (%) | 61.5 ± 6.6 | 61.4 ± 6.6 | 61.6 ± 6.6 | 0.578 |
| Laboratory indicators | ||||
| Hemoglobin (g/l) | 124.2 ± 20.9 | 139.9 ± 13.8 | 109.0 ± 14.3 | <0.001 |
| eGFR (ml·min−1·1.73 m−2) | 71.8(51.2,92.2) | 78.1(61.1,97.0) | 64.0(43.2,86.7) | <0.001 |
| eGFR <60 ml·min−1·1.73 m−2 | 562(35.0%) | 190(24.2%) | 372(45.5%) | <0.001 |
| Serum sodium (mmol/l) | 140.1 ± 6.6 | 140.3 ± 6.3 | 139.9 ± 6.8 | 0.242 |
| Serum potassium (mmol/l) | 4.0 ± 0.6 | 3.9 ± 0.5 | 4.0 ± 0.6 | 0.034 |
| TC (mmol/l) | 4.3 ± 2.2 | 4.3 ± 1.7 | 4.2 ± 2.5 | 0.168 |
| HDL (mmol/l) | 1.2 ± 0.5 | 1.2 ± 0.4 | 1.2 ± 0.6 | 0.795 |
| LDL (mmol/l) | 2.4 ± 0.9 | 2.5 ± 0.9 | 2.3 ± 0.9 | <0.001 |
| TG (mmol/l) | 1.2(0.9,1.7) | 1.3(1.0,1.8) | 1.1(0.8,1.6) | <0.001 |
| BNP (ng/l) | 329.0(145.0,748.1) | 313.4(145.9,709.8) | 331.5(143.8,761.8) | 0.431 |
| NT-proBNP (ng/l) | 1094.0(453.0,2666.5) | 831.9(387.1,1940.2) | 1569.5(581.7,3561.7) | <0.001 |
| BNP > 400 ng/l or NT-proBNP > 1500 ng/l | 685(42.7%) | 272(34.6%) | 413(50.5%) | <0.001 |
| Current medication | ||||
| ACEI/ARB | 1156(72.1%) | 602(76.6%) | 554(67.7%) | <0.001 |
| Beta-blockers | 1040(64.8%) | 527(67.0%) | 513(62.7%) | 0.069 |
| Digital | 423(26.4%) | 202(25.7%) | 221(27.0%) | 0.549 |
| MRAs | 1087(67.8%) | 500(63.6%) | 587(71.8%) | <0.001 |
| Nitrates | 862(53.7%) | 401(51.0%) | 461(56.4%) | 0.032 |
| Stain | 840(52.4%) | 445(56.6%) | 395(48.3%) | 0.001 |
| Aspirin | 839(52.3%) | 449(57.1%) | 390(47.7%) | <0.001 |
| Anticoagulant therapy | 128(8.0%) | 73(9.3%) | 55(6.7%) | 0.058 |
| Traditional Chinese medicine | 374(23.3%) | 175(22.3%) | 199(24.3%) | 0.329 |
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; Na, sodium; K, Potassium; TC, serum total cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglyceride; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonist.
1416 (88.3%) patients completed the endpoint follow-up. The median follow-up duration was 33.9 months (interquartile range 23.1–40.8 months), incidence of death (17.5% vs. 10.6%) and re-hospitalization (44.9% vs. 37.7%) was higher in anemia group than in non-anemia group. Univariate COX proportional regression showed that anemia (HR 1.74, 95% CI 1.33–2.28, p < 0.001) and RD (HR 1.76, 95% CI 1.36–2.29, p < 0.001) were significantly associated with increased all-cause mortality risk, however, after adjustment for baseline age, heart rate, serum sodium, serum potassium, TC, HDL, LDL, TG, NYHA class, BNP/NT-proBNP, ACEI/ARB, digital, MRA, stain and aspirin, multivariate COX model found that anemia and renal function were not independent predictors of all-cause mortality risk (Table 2). When analyzing the risk factors for all-cause re-hospitalization, univariate Cox regression indicated that the HR associated with anemia was 1.27 (95%CI 1.09–1.47, p = 0.003), and the HR associated with RD was 1.17 (95%CI 0.99–1.36, p = 0.054); similarly, the adjusted multivariate Cox regression showed that anemia (HR 1.13, 95% CI 0.96–1.33, p = 0.152) and RD (HR 0.94, 95% CI 0.79–1.12, p = 0.488) were not statistically associated with all-cause re-hospitalization risk.
Table 2.
COX proportional regression analysis associated with all-cause mortality.
| Univariate cox regression |
Multivariate cox regression |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p value | HR | 95%CI | p value | |
| Anemia | 1.74 | 1.33–2.28 | <0.001 | 1.14 | 0.85–1.52 | 0.386 |
| RD | 1.76 | 1.36–2.29 | <0.001 | 1.18 | 0.88–1.57 | 0.269 |
| Age | 1.07 | 1.05–1.08 | <0.001 | 1.05 | 1.03–1.07 | <0.001 |
| Heart rate | 1.01 | 1.00–1.02 | 0.006 | 1.01 | 0.99–1.01 | 0.117 |
| Serum sodium | 0.99 | 0.98–1.00 | 0.003 | 0.98 | 0.97–0.99 | 0.001 |
| Serum potassium | 1.40 | 1.13–1.74 | 0.002 | 1.22 | 0.98–1.52 | 0.071 |
| TC | 0.90 | 0.80–1.01 | 0.062 | 0.99 | 0.90–1.09 | 0.860 |
| HDL | 1.40 | 1.21–1.63 | <0.001 | 1.34 | 1.11–1.61 | 0.002 |
| LDL | 0.84 | 0.72–0.98 | 0.028 | 0.90 | 0.75–1.07 | 0.220 |
| TG | 0.62 | 0.50–0.78 | <0.001 | 0.82 | 0.66–1.01 | 0.060 |
| NYHA class | 1.80 | 1.50–2.16 | <0.001 | 1.42 | 1.15–1.74 | 0.001 |
| BNP > 400 ng/l or NT-proBNP > 1500 ng/l | 2.05 | 1.57–2.67 | <0.001 | 1.26 | 0.93–1.70 | 0.131 |
| ACEI/ARB | 0.71 | 0.54–0.94 | 0.018 | 0.81 | 0.61–1.08 | 0.144 |
| Digital | 1.68 | 1.28–2.20 | <0.001 | 1.27 | 0.94–1.72 | 0.121 |
| MRA | 1.76 | 1.28–2.42 | 0.001 | 0.98 | 0.69–1.39 | 0.887 |
| Stain | 0.74 | 0.57–0.96 | 0.024 | 1.01 | 0.75–1.36 | 0.966 |
| Aspirin | 0.78 | 0.60–1.02 | 0.067 | 1.05 | 0.78–1.40 | 0.765 |
RD, renal dysfunction; TC, serum total cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglyceride; NYHA, New York Heart Association; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonist.
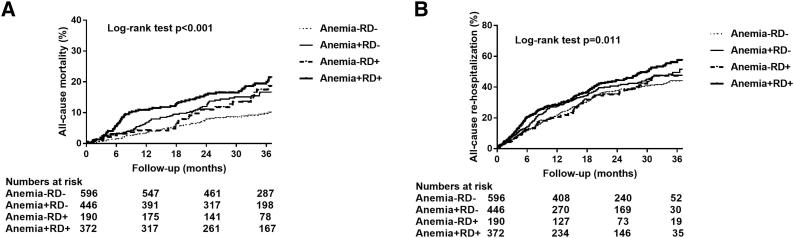
There were 596 (37.2%) patients in the Anemia−RD− group, 446 (27.8%) in the Anemia+RD− group, 190 (11.8%) in the Anemia−RD+ group and 372 (23.2%) patients in the Anemia+RD+ group. Kaplan-Meier survival curves and log-rank test showed a significant difference both in all-cause mortality (p < 0.001) and in all-cause re-hospitalization (p = 0.011) among the four groups (Fig. 2). Compared with the reference group (Anemia+RD+), the adjusted HR in the Anemia−RD− group, Anemia+RD− group and Anemia−RD+ group for all-cause mortality were 0.74 (95% CI 0.50–1.08, p = 0.121), 0.92 (95% CI 0.65–1.31, p = 0.640) and 1.00 (95% CI 0.65–1.54, p = 1.000); the HR in the Anemia−RD− group, Anemia+RD− group and Anemia−RD+ group for all-cause re-hospitalization risk were 0.94 (95% CI 0.76–1.17, p = 0.567), 1.02 (95% CI 0.82–1.26, p = 0.872) and 0.82 (95% CI 0.63–1.09, p = 0.170) (Table 3).
Fig. 2.
Kaplan-Meier survival curve illustrating all-cause mortality (A) and all-cause re-hospitalization (B) on different groups. Anemia−RD−, patients with neither anemia nor renal dysfunction; Anemia+RD−, patients with anemia but no renal dysfunction; Anemia-RD+, patients with renal dysfunction but no anemia, Anemia+RD+, patients with both anemia and renal dysfunction;
Table 3.
Hazard Ratios and 95%CI of anemia associated with cardiac endpoints, classified with renal dysfunction.
| Numbers of events n (%) | HR | 95%CI | p value | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Anemia−RD− | 52(8.7%) | 0.74 | 0.50–1.08 | 0.121 |
| Anemia+RD− | 65(14.6%) | 0.92 | 0.65–1.31 | 0.640 |
| Anemia−RD+ | 31(16.3%) | 1.00 | 0.65–1.54 | 1.000 |
| Anemia+RD+ | 78(21.0%) | 1 | – | – |
| All-cause re-hospitalization | ||||
| Anemia−RD− | 223(37.4%) | 0.94 | 0.76–1.17 | 0.567 |
| Anemia+RD− | 186(41.7%) | 1.02 | 0.82–1.26 | 0.872 |
| Anemia−RD+ | 73(38.4%) | 0.82 | 0.63–1.09 | 0.170 |
| Anemia+RD+ | 181(48.7%) | 1 | – | – |
Anemia−RD−, patients with neither anemia nor renal dysfunction; Anemia+RD−, patients with anemia but no renal dysfunction; Anemia−RD+, patients with renal dysfunction but no anemia, Anemia+RD+, patients with both anemia and renal dysfunction.
When the association was examined after stratifying by NYHA class (NYHA I-II/NYHA III-IV), incidence of death was 8.3% and re-hospitalization was 34.8% in NYHA class I-II group, and 19.2% and 47.2% in NYHA class III-IV group, respectively. Anemia was an independent predictor of risk for all-cause re-hospitalization (HR 1.32, 95% CI 1.07–1.65, p = 0.011) in patients with NYHA class III-IV, but not in patients with NYHA class I-II.
4. Discussion
This study showed that the prevalence of anemia in HFpEF patients was 51.0% in CN-HF registry, and anemia was not independently associated with all-cause mortality and all-cause re-hospitalization, except for the all-cause re-hospitalization in NYHA class III-IV patients.
The prevalence of anemia in HFpEF patients varies greatly from 15.0% to 79.0% in different reports of different region, different study population, different LVEF and different diagnostic criteria of anemia [2,[12], [13], [14], [15], [16], [17], [18], [19], [20]] (Table 4). The prevalence of anemia in this study was roughly consistent with previous studies.
Table 4.
Prevalence of anemia in patients with HFpEF in different studies.
| Numbers of patients | LVEF | Diagnostic criteria for anemia | Prevalence (%) | |
|---|---|---|---|---|
| Parissis et al. [12] | 837 | ≥45% | 15.0 | |
| Tsuchihashi-Makaya et al. [13] | 429 | ≥50% | 27.1 | |
| Goyal et al. [14] | 2,330,361 | 33.6 | ||
| Abohammar et al. [15] | 109 | >50% | 35.0 | |
| Donal et al. [16] | 539 | >45% | WHO | 37.0 |
| Tarantini et al. [17] | 726 | >40% | Hb < 120 g/l | 37.0 |
| Felker et al. [18] | 3093 | >40% | WHO | 38.0 |
| Tada et al. [19] | 357 | WHO | 39.0 | |
| CN-HF | 1604 | ≥50% | WHO | 51.0 |
| Katsuya Kajimoto, et al. [2] | 2210 | >40% | WHO | 53.4 |
| Saheb Sharif-Askari et al. [20] | 106 | WHO | 79.0 |
World Health Organization (WHO): anemia was defined as a hemoglobin (Hb) <130 g/l for men and <120 g/l for women.
The impact of anemia on clinical outcomes in patients with HFpEF has been investigated in previous studies. Brucks et al. [21] showed that anemia did not significantly reduce the survival (p = 0.09) in 137 patients with diastolic heart failure (LVEF ≥ 50%). The Italian Acute Heart Failure Survey (IS-AHF) found that anemia (Hb < 120 g/l) did not significantly increase the all-cause mortality during hospitalization in patients with HFpEF (p = 0.08) [17]. The ESC-HF Pilot Survey also concluded that anemia was not an independent predictor of all-cause death and the composite of all-cause death and re-hospitalization for HF at 1 year [22].
However, controversial results were reported by other research groups. Felker GM et al. [18] reported for the first time that anemia was an independent predictor of all-cause mortality in 3093 patients with HFpEF (LVEF > 40%) at Duke University Medical Center in the United States. A retrospective study found that anemia was associated with increased 5 years mortality rates rather than all-cause hospitalization rates and cardiac-related hospitalization rates in 295 patients who have diastolic heart failure (LVEF ≥ 50%) [23]. Halawa A et al. [24] showed that anemia (Hb < 100 g/l) was associated with 3-year mortality in patients with acute heart failure. In recent years, some subgroup analysis also agreed with this conclusion [2,25]. In these studies, Felker GM et al. included patients of heart failure with mid-range ejection fraction (HFmEF), Halawa A et al. targeted only at patients with acute heart failure, and the definition of anemia was more stringent.
The impacts of anemia in patients with might be multifactorial. Direct cardiac hypoxia, increased oxidative stress, increased fluid retention, increased sympathetic, and renin-angiotensin aldosterone activity [26] might all be related to anemia-induced negative effects in heart failure patients. It is known that anemia might decrease the oxygen-carrying capacity of the blood, which is mainly compensated by the increase of heart rate and stroke volume. Since the effect of increased heart rate is greater on diastolic period than that on systolic period, the increased heart rate and subsequent increase on cardiac output may partly explain why anemia did not resulted in poorer outcomes in HFpEF patients with anemia as compared to HFpEF patients without anemia [[27], [28], [29]].
Both anemia and RD are common complications of heart failure. In our study, prevalence of renal dysfunction is also higher in patients with anemia (Table 1). Postulated mechanisms include reduced production of erythropoietin due to RD, antiproliferative effects of accumulated uremia toxins, changes in iron homeostasis, and chronic immune activation [30,31]. Our results further showed that Anemia-RD+ group and Anemia+RD+ group had similar all-cause mortality, which was consistent with the subgroup analysis of the Norwegian Heart Failure Registry and concluded that baseline anemia was not an independent predictor of all-cause mortality in outpatients with severe renal insufficiency [32]. However, the ANCHOR Study found the anemia (120 ≤ Hb < 130, 110 ≤ Hb < 120, 100 ≤ Hb < 110, 90 ≤ Hb < 100) and renal dysfunction (30 ≤ GFR < 44, 15 ≤ GFR < 30, GFR < 15) independently increased the risk of death from any cause, and hemoglobin level was an independent predictor of outcomes at all levels of kidney function, regardless of the level of systolic function [33]. The differences are considered in the following three aspects: First of all, our study population were limited to the hospitalized HFpEF patients. Secondly, we grouped the patients into anemia and renal dysfunction according to admission measurements of hemoglobin and eGFR values, and others further divided the patients into more detailed subgroups according to the degree of anemia and RD [33]. Accordingly, both anemia and renal dysfunction defined in our study may be transitory rather than chronic, these factors should be considered when interpreting the data presented in this study.
In addition, results from previous subgroup studies of HFrEF or heart failure have found that anemia can increase the risk of all-cause mortality in NYHA class I-II patients [2,34]. Thus, we also performed a subgroup analysis of different NYHA class in patients with HFpEF. The results were different from the results of patients with HFrEF or heart failure [2,34].
Two study limitations of our study need to be mentioned: Firstly, multivariate COX regression is used to control confounding bias as much as possible that may affect outcome, but it cannot control the variables that are not measured, The results need to be confirmed in a randomized controlled trial; Secondly, CN-HF included both acute and chronic heart failure patients, but there was no record of acute and chronic heart failure classification, therefore, we cannot analysis the impact of anemia on acute and chronic patients with HFpEF.
5. Conclusions
In summary, the prevalence of anemia in hospitalized patients with HFpEF was 51.0% in CN-HF. Anemia was not an independent predictor of all-cause mortality and re-hospitalization when NYHA class was not considered, meanwhile, there was no interaction between anemia and renal dysfunction in predicting the endpoint event.
Acknowledgments
Acknowledgements
We would like to acknowledge all the investigators in the research sites for making data available for public use.
-
1.
Qiaoqing Zhong. Chenzhou No.1 People's Hospital, Chenzhou, China.
-
2.
Yingmin Lu. Xin Hua Hospital affiliated to Shanghai Jiao Tong University School of Medicine Chongming branch, Shanghai, China.
-
3.
Guohui Zhang. Zhenjiang First People's Hospital, Zhenjiang, China.
-
4.
Li Chen. Affiliated Hospital of North Sichuan Medical College, Nanchong, China.
-
5.
Huimin Gu. First People's Hospital of Kunshan, Kunshan, China.
-
6.
Meng Wei. Shanghai Sixth People's Hospital, Shanghai, China.
-
7.
Lianglong Chen. Fujian Medical University Union Hospital, Fuzhou, China.
-
8.
Jun Wang. Jing'an District Central Hospital of Shanghai, Shanghai, China.
-
9.
Zhenyu Yang. Wuxi People's Hospital, Wuxi, China.
-
10.
Huigen Jin. Putuo People's Hospital of Shanghai, Shanghai, China.
-
11.
Xianliang Li. Zhangjiagang Hospital of Traditional Chinese Medicine, Zhangjiagang, China.
-
12.
Zengyong Qiao. Shanghai Fengxian District Central Hospital, Shanghai, China.
-
13.
Yingjun Yang. Jiashan First People's Hospital, Jiashan, China.
-
14.
Yulan Zhao. The Second Affiliated Hospital of Zhenzhou University, Zhengzhou, China.
-
15.
Ru Jia. The No.4 Hospital 1946 Jinan Shandong, Ji'nan, China.
-
16.
Bin Hong. Shanghai Qingpu District Central Hospital, Shanghai, China.
-
17.
FangYuan. Shanghai Chest Hospital, Shanghai, China.
-
18.
Jufei Wang. Fenghua Hospital, Fenghua, China.
-
19.
Jin Ma. Yangpu Hospital, Tongji University, Shanghai, China.
-
20.
Yu Xu. Henan Provincial People's Hospital, Zhengzhou, China.
-
21.
Liwen Li. Guangdong General Hospital, Guangzhou, China.
-
22.
Mahmud Wahafu. The Second People's Hospital of Kashgar Prefecture, Kashi, Xinjiang, China.
-
23.
Qin Yu. Zhongshan Hospital, Dalian University,Dalian, China.
-
24.
Changqian Wang. Shanghai Ninth People's Hospital, Shanghai, China.
-
25.
Changwu Ruan. Shanghai Eighth People's Hospital, Shanghai, China.
-
26.
Honggang Fu. Fuyuan People's Hospital, Qujing, China.
-
27.
Xuebo Liu. Shanghai East Hospital, Tongji University, Shanghai, China.
-
28.
Xin Xu. Wuxi No.2 People's Hospital, Wuxi, China.
-
29.
Shaoping Chen. Changhai Hospital of Shanghai, Shanghai, China.
-
30.
Qiliang Liu. Putuo District Center Hospital, Shanghai, China.
-
31.
Bei Shi. Hospital of Zhunyi Medical College, Zunyi, China.
-
32.
Jiahong Xu. Tongji Hospital, Tongji University, Wuhan, China.
-
33.
Zhenyue Chen. Rui Jin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Sources of funding
This study was supported by the National Science & Technology Pillar Program, 12th 5-year plan of China (2011BAI11B10).
Declaration of competing interest
None.
Contributor Information
Jingmin Zhou, Email: zhou.jingmin@zs-hospital.sh.cn.
Junbo Ge, Email: jbge@zs-hospital.sh.cn.
References
- 1.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Revista espanola de cardiologia (English ed) 2016;69(12):1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Goh V.J., Tromp J., Teng T.K., Tay W.T., Van Der Meer P., Ling L.H. Prevalence, clinical correlates, and outcomes of anaemia in multi-ethnic Asian patients with heart failure with reduced ejection fraction. ESC heart failure. 2018;5(4):570–578. doi: 10.1002/ehf2.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajimoto K., Sato N., Takano T. Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur. Heart J. Acute Cardiovasc. Care. 2015;4(6):568–576. doi: 10.1177/2048872614554199. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ahmad A., Rand W.M., Manjunath G., Konstam M.A., Salem D.N., Levey A.S. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 2001;38(4):955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 5.Kyriakou M., Kiff P.F. Prognosis of the comorbid heart failure and Anemia: a systematic review and meta-analysis. Clinical Trials and Regulatory Science in Cardiology. 2016;16:12–21. [Google Scholar]
- 6.Jankowska E.A., Drozd M., Ponikowski P. Handbook of Experimental Pharmacology. vol. 243. 2017. Iron deficiency treatment in patients with heart failure; pp. 561–576. [DOI] [PubMed] [Google Scholar]
- 7.Yancy C.W., Lopatin M., Stevenson L.W., De Marco T., Fonarow G.C. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J. Am. Coll. Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg B.A., Zhao X., Heidenreich P.A., Peterson E.D., Bhatt D.L., Cannon C.P. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 9.Chinese guidelines for the diagnosis and treatment of heart failure 2014Zhonghua xin xue guan bing za zhi. 2014;42(2):98–122. [PubMed] [Google Scholar]
- 10.Ma Y.C., Zuo L., Chen J.H., Luo Q., Yu X.Q., Li Y. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 11.Nutritional anaemias. Report of a WHO scientific group. vol. 405. 1968. pp. 5–37. (World Health Organization technical report series). [PubMed] [Google Scholar]
- 12.Parissis J.T., Ikonomidis I., Rafouli-Stergiou P., Mebazaa A., Delgado J., Farmakis D. Clinical characteristics and predictors of in-hospital mortality in acute heart failure with preserved left ventricular ejection fraction. Am. J. Cardiol. 2011;107(1):79–84. doi: 10.1016/j.amjcard.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchihashi-Makaya M., Hamaguchi S., Kinugawa S., Yokota T., Goto D., Yokoshiki H. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73(10):1893–1900. doi: 10.1253/circj.cj-09-0254. [DOI] [PubMed] [Google Scholar]
- 14.Goyal P., Almarzooq Z.I., Horn E.M., Karas M.G., Sobol I., Swaminathan R.V. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am. J. Med. 2016;129(6) doi: 10.1016/j.amjmed.2016.02.007. (635.e15–26) [DOI] [PubMed] [Google Scholar]
- 15.Abohammar S., ElSaidy M.A., Fathalla D., Aldosarri M. Baseline characteristics of patients with heart failure and preserved ejection fraction at admission with acute heart failure in Saudi Arabia. The Egyptian Heart Journal. 2017;69(1):21–28. doi: 10.1016/j.ehj.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donal E., Lund L.H., Oger E., Hage C., Persson H., Reynaud A. Baseline characteristics of patients with heart failure and preserved ejection fraction included in the Karolinska Rennes (KaRen) study. Archives of Cardiovascular Diseases. 2014;107(2):112–121. doi: 10.1016/j.acvd.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Tarantini L., Oliva F., Cantoni S., Cioffi G., Agnoletto V., Alunni G. Prevalence and prognostic role of anaemia in patients with acute heart failure and preserved or depressed ventricular function. Intern. Emerg. Med. 2013;8(2):147–155. doi: 10.1007/s11739-011-0601-z. [DOI] [PubMed] [Google Scholar]
- 18.Felker G.M., Shaw L.K., Stough W.G., O'Connor C.M. Anemia in patients with heart failure and preserved systolic function. Am. Heart J. 2006;151(2):457–462. doi: 10.1016/j.ahj.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Tada T., Shiba N., Watanabe J., Matsuki M., Kagaya Y., Shinozaki T. Prognostic value of anemia in predicting sudden death of patients with diastolic heart failure. Int. J. Cardiol. 2008;128(3):419–421. doi: 10.1016/j.ijcard.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 20.Saheb Sharif-Askari N., Sulaiman S.A., Saheb Sharif-Askari F., Al Sayed Hussain A., Tabatabai S., Al-Mulla A.A. Hospitalized heart failure patients with preserved vs. reduced ejection fraction in Dubai, United Arab Emirates: a prospective study. Eur. J. Heart Fail. 2014;16(4):454–460. doi: 10.1002/ejhf.51. [DOI] [PubMed] [Google Scholar]
- 21.Brucks S., Little W.C., Chao T., Rideman R.L., Upadhya B., Wesley-Farrington D. Relation of anemia to diastolic heart failure and the effect on outcome. Am. J. Cardiol. 2004;93(8):1055–1057. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Tyminska A., Kaplon-Cieslicka A., Ozieranski K., Peller M., Balsam P., Marchel M. Anemia at hospital admission and its relation to outcomes in patients with heart failure (from the Polish Cohort of 2 European Society of Cardiology Heart Failure Registries) Am. J. Cardiol. 2017;119(12):2021–2029. doi: 10.1016/j.amjcard.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Tehrani F., Phan A., Morrissey R., Chien C., Rafique A., Schwarz E.R. The prognostic value of anemia in patients with diastolic heart failure. Tex. Heart Inst. J. 2009;36(3):220–225. [PMC free article] [PubMed] [Google Scholar]
- 24.Halawa A., Burton M.C., Maniaci M.J., Shapiro B.P., Yip D.S., Hodge D.O. Association of anemia with outcomes of acute heart failure. South. Med. J. 2018;111(2):103–108. doi: 10.14423/SMJ.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 25.Berry C., Poppe K.K., Gamble G.D., Earle N.J., Ezekowitz J.A., Squire I.B. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: results from the MAGGIC individual patient data meta-analysis. QJM: Monthly Journal of the Association of Physicians. 2016;109(6):377–382. doi: 10.1093/qjmed/hcv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverberg D., Wexler D., Blum M., Wollman Y., Iaina A. The cardio-renal anaemia syndrome: does it exist? Nephrol. Dial. Transplant. 2003;18(Suppl. 8):viii7–12. doi: 10.1093/ndt/gfg1084. [DOI] [PubMed] [Google Scholar]
- 27.Anand I.S., Chandrashekhar Y., Ferrari R., Poole-Wilson P.A., Harris P.C. Pathogenesis of oedema in chronic severe anaemia studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br. Heart J. 1993;70(4):357–362. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand I.S., Chandrashekhar Y., Wander G.S., Chawla L.S. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J. Am. Coll. Cardiol. 1995;25(6):1402–1407. doi: 10.1016/0735-1097(95)00007-Q. [DOI] [PubMed] [Google Scholar]
- 29.Katz S.D., Rao R., Berman J.W., Schwarz M., Demopoulos L., Bijou R. Pathophysiological correlates of increased serum tumor necrosis factor in patients with congestive heart failure. Relation to nitric oxide-dependent vasodilation in the forearm circulation. Circulation. 1994;90(1):12–16. doi: 10.1161/01.cir.90.1.12. [DOI] [PubMed] [Google Scholar]
- 30.Eschbach J.W. Anemia management in chronic kidney disease: role of factors affecting epoetin responsiveness. J Am Soc Nephrol. 2002;13(5):1412–1414. doi: 10.1097/01.asn.0000016440.52271.f7. [DOI] [PubMed] [Google Scholar]
- 31.Weiss G., Goodnough L.T. Anemia of chronic disease. N. Engl. J. Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 32.Waldum B., Westheim A.S., Sandvik L., Flonaes B., Grundtvig M., Gullestad L. Baseline anemia is not a predictor of all-cause mortality in outpatients with advanced heart failure or severe renal dysfunction. Results from the Norwegian Heart Failure Registry. J. Am. Coll. Cardiol. 2012;59(4):371–378. doi: 10.1016/j.jacc.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 33.Go A.S., Yang J., Ackerson L.M., Lepper K., Robbins S., Massie B.M. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson A., Hallberg A.C., Edner M., Lund L.H., Dahlstrom U. A comprehensive assessment of the association between anemia, clinical covariates and outcomes in a population-wide heart failure registry. Int. J. Cardiol. 2016;211:124–131. doi: 10.1016/j.ijcard.2016.02.144. [DOI] [PubMed] [Google Scholar]