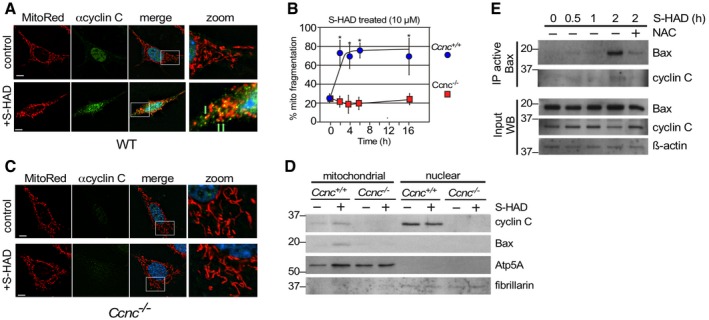
Wild‐type MEF cells were fixed before (control) and following S‐HAD treatment (10 μM, 4 h); then, nuclei (DAPI), cyclin C (indirect immunofluorescence), and mitochondria (MitoTracker Red) were visualized by fluorescence microscopy. Merge panels present DAPI staining (blue) only. Zoom panels (4×) are indicated by the boxes in the merge panels. Green arrows indicate cyclin C and mitochondrial co‐localization. Bar indicates 10 μM.
Timecourse experiment with wild‐type and Ccnc
−/− MEF cells treated with S‐HAD (10 μM) for the times indicated (n = 3). Error bars indicate SD. Asterisks indicate P values < 0.05 from pretreatment control (Student's t‐test).
S‐HAD peptide activity requires cyclin C. The experiment described in (A) was repeated with Ccnc
−/− MEF cultures. Bar indicates 10 μm. Results quantitated in (B).
S‐HAD treatment increases Bax mitochondrial localization. Western blot analysis of the indicated proteins in nuclear and mitochondrial fractions before and following S‐HAD treatment (10 μM, 4 h). Atp5A and fibrillarin levels controlled for mitochondrial and nuclear loadings, respectively. Molecular weight markers (kDa) are indicated on the left.
Bax activation was monitored in wild‐type MEF cultures treated with S‐HAD (10 μM) for the times indicated. The anti‐oxidant N‐acetyl cysteine (NAC) was added (1 mM) 2 h prior to S‐HAD treatment. Active Bax immunoprecipitates were subjected to Western blot analysis for Bax and cyclin C as indicated. Bax, cyclin C, and β‐actin levels were monitored in extract preparations as input controls. Molecular weight markers (kDa) are indicated on the left.