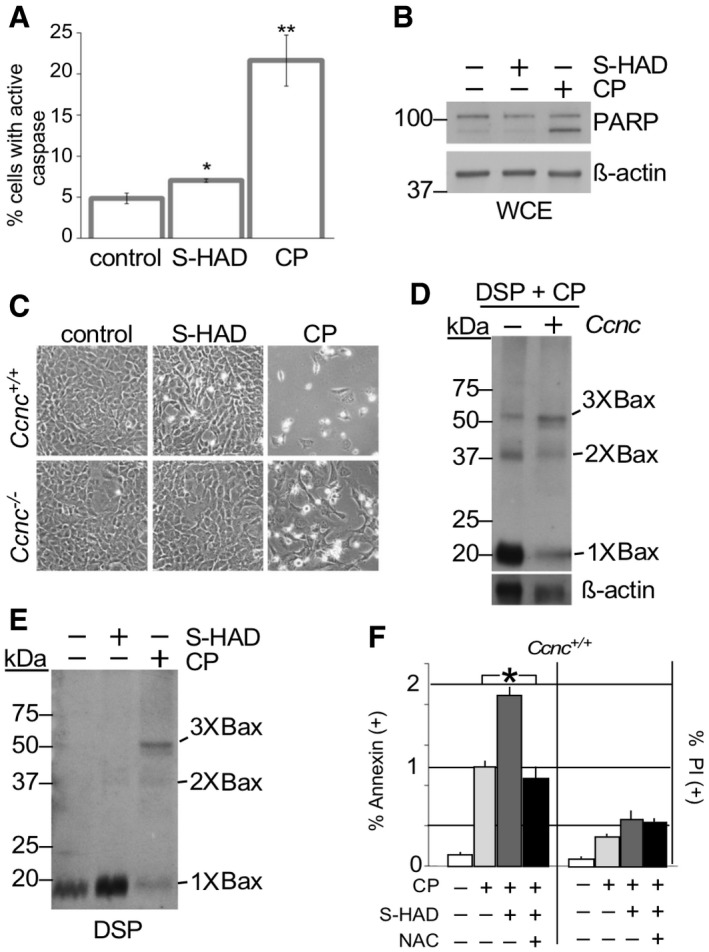
Caspase activation was measured by fluorescent cell analysis in wild‐type MEF cells treated with S‐HAD (10 μM, 24 h) or cisplatin (CP, 30 μM, 16 h). n = 3. Error bars indicate SD. * and ** indicate P < 0.05 and P < 0.01, respectively, using Student's t‐test.
PARP cleavage was not induced by S‐HAD treatment. PARP cleavage was monitored by Western blot analysis in HeLa cells treated with S‐HAD or cisplatin (CP) as described in (A). The blot was stripped and reprobed with β‐actin antibodies as a loading control. Molecular weight markers (kDa) are indicated on the left.
Wild‐type and
Ccnc
−/− MEF cultures were treated with S‐HAD and cisplatin (CP) as just described. Cells were imaged and examined for a “rounding up” phenotype diagnostic for death (see Fig
EV4A for quantitation).
Cyclin C is required for normal Bax oligomerization in response to cisplatin. Whole cell extracts prepared from DSP crosslinked wild‐type and CCNC
−/− MEF cells exposed to cisplatin (30 μM, 16 h) were probed for total Bax. Bax multimers are indicated. Molecular weight markers (kDa) are indicated on the left.
S‐HAD does not induce Bax oligomerization. Experiments described in (D) were repeated with cultures treated with S‐HAD (10 μM, 24 h). Bax multimers are indicated. Molecular weight markers (kDa) are indicated on the left.
S‐HAD treatment sensitizes Hela cells to cisplatin. Hela cultures were treated with cisplatin (30 μM), S‐HAD (10 μM), and N‐acetyl cysteine (NAC, 1 mM) as indicated. Fluorescent cell analysis was used to quantitate the apoptotic (Annexin V positive) and necrotic (PI positive) population percentages, respectively. n = 3 independent cultures. Error bars indicate SD. Asterisk indicates P < 0.05 (Student's t‐test).