Figure 2.
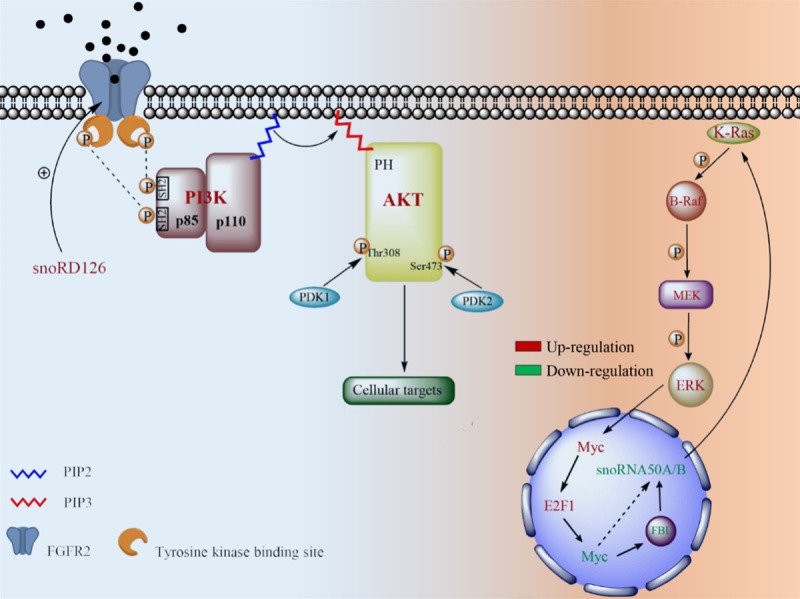
SnoRD126 activates the PI3K-AKT signaling pathway through FGFR2 and snoRD50A/B and affects the MAPK signaling cascades. The expression level of snoRD126 is positively correlated with FGFR2. It contains tyrosine kinase residues which are auto-phosphorylated through binding its ligand. Being of activated, the phosphorylated residues on tyrosine kinase binding site provide binding sites for the SH2 domain of p85. Activation of PI3K induces an alteration of PIP2 to PIP3. PIP3 functions as a second messenger when it is combined with the PH domain of AKT, leading to activation of AKT. The activation is also required members of AGC protein kinase family-PDK1 (phosphoinositide dependent kinase-1) and PDK2 (phosphoinositide dependent kinase-2) which phosphorylate residues on Thr308 and Ser473 of AKT protein, respectively. The deletion of snoRD50A/B increases the binding of GTP and K-Ras, leading to the activation of K-Ras. The activation of K-Ras activates the mitogen-activated protein kinase (MAPK) signaling cascades. It is consisted of the MAP kinase kinase kinase (MAP3K), the MAP kinase kinase (MAP2K) and MAPK. They are phosphorylated in cascades order, thereby transmitting upstream signals to downstream responders.
