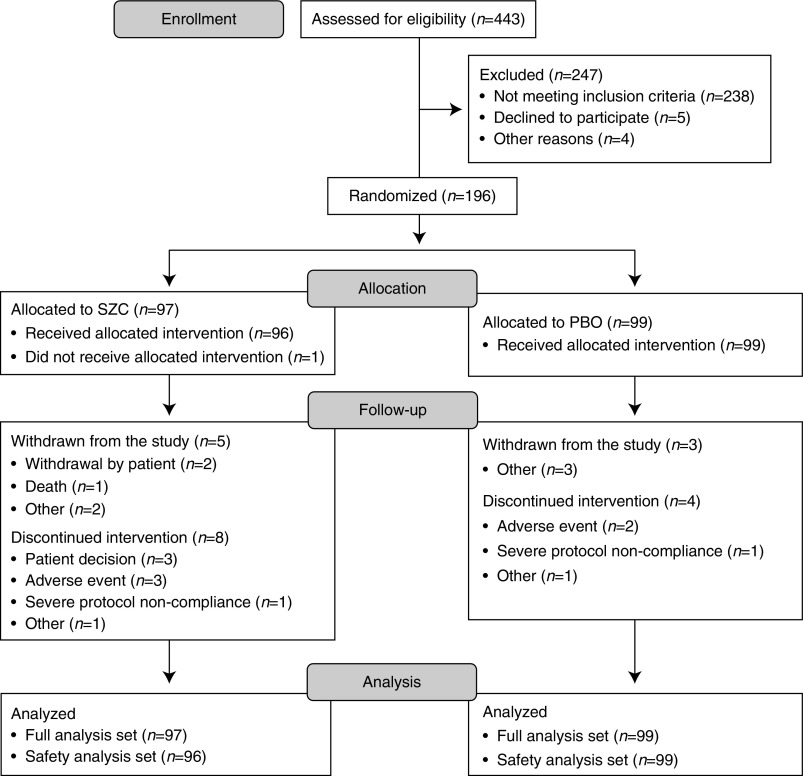
Figure 2.
Flow diagram of patient disposition. “Other” reasons for withdrawal from study for sodium zirconium cyclosilicate (SZC): adverse event (n=1) and transferred to a nonstudy dialysis center (n=1). “Other” reasons for withdrawal from study for placebo (PBO): kidney transplantation (n=1), discontinuation of investigational product (n=1), and noncompliance to study protocol (n=1). “Other” reasons for discontinuation of intervention for SZC: transferred to a nonstudy dialysis center (n=1). “Other” reasons for discontinuation of intervention for PBO: patient hospitalized due to nontreatment-related serious adverse event and was unable to receive treatment during hospitalization and until end of treatment visit (n=1).