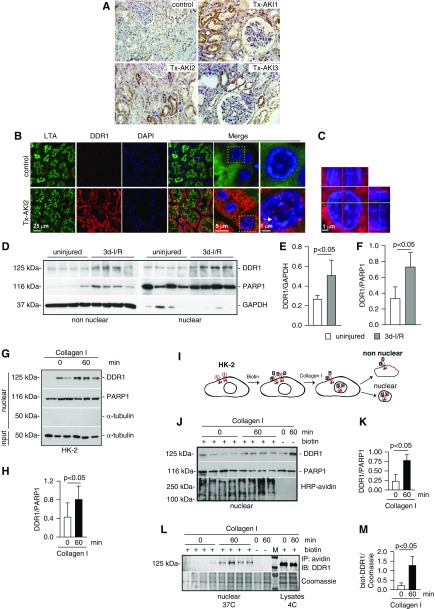
Figure 1.
DDR1 is upregulated in injured kidney proximal tubules. (A) Paraffin kidney sections from control and biopsy specimens from three different patients with transplant AKI (Tx-AKI) were stained with anti-DDR1 antibody. Upregulated DDR1 expression is evident in the tubules of injured kidneys. (B) Paraffin kidney sections from control or one patient with Tx-AKI were stained with anti-DDR1 antibody and Lotus tetragonolobus agglutinin (LTA, a marker of proximal tubule) and analyzed by confocal microscopy. Expression of DDR1 is evident both in the cytoplasm and in the nuclei of injured proximal tubules (arrow). (C) Orthogonal projection of confocal images of kidney sections from the patient shown in (B) was performed using the imaging program Zen (black edition). Red, DDR1; blue, DAPI. (D) Non-nuclear and nuclear fractions (20 µg/lane) from kidney cortices of wild-type mice uninjured or 3 days after ischemia-reperfusion (3d-I/R) were analyzed by western blot for levels of DDR1. (E and F) Non-nuclear DDR1 and GAPDH (E) or nuclear DDR1 and PARP1 (F) bands were quantified by densitometry. Values represent DDR1/GAPDH or DDR1/PARP1 ratio and are the mean±SD of four animals. (G) Serum-starved HK-2 cells were treated with collagen I (50 µg/ml) for the time indicated. Time 0 represents cells incubated with vehicle (20 mM acetic acid) for 60 minutes. Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of DDR1. (H) Nuclear DDR1 and PARP1 bands were quantified by densitometry. Values represent DDR1/PARP1 ratio and are the mean±SD of two experiments performed in triplicate. PARP1 (nuclear marker), GAPDH, or α-tubulin (non-nuclear markers) was used to evaluate fraction purity. (I) Schematic representation of the biotinylation assay performed on HK-2 cells. See text for details. (J) Nuclear fractions of HK-2 cells biotinylated (+ biotin) and treated at 37°C with collagen I for the time indicated were analyzed for levels of DDR1 or total biotinylated proteins using HRP-avidin. Nonbiotinylated (- biotin) cells treated with collagen I for the times indicated served as control. (K) Nuclear DDR1 and PARP1 of biotinylated cells were quantified and expressed as indicated above. (L) Nuclear fractions (200 µg) of biotinylated HK-2 cells treated at 37°C with collagen I for the times indicated were immunoprecipitated using streptavidin beads. Immunoprecipitated biotinylated proteins were analyzed for levels of DDR1. Cells treated at 37°C with collagen I for the time indicated in the absence of biotinylation (- biotin) or biotinylated by kept at 4°C served as negative (background for streptavidin beads) and positive (total biotinylated DDR1) controls, respectively. (M) Nuclear biotinylated DDR1 was quantified to the Coomassie protein band shown. IP, immunoprecipitation; IB, immunoblot.