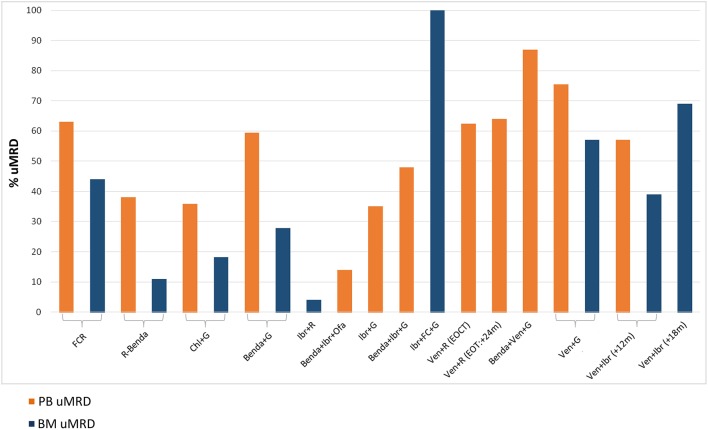
Figure 2.
Percentages of undetectable MRD (uMRD) in peripheral blood (PB) and bone marrow (BM) compartments across different clinical trials. On the Left, the standard chemoimmunotherapy for CLL patients. On the Right, the most recent clinical trials incorporating BCR/BCL2 inhibitors. FCR, fludarabine+cyclophosphamide+rituximab (CLL8) (14, 81); R-Benda, rituximab+bendamustine (CLL10) (82); Chl+G, chlorambucil+obinutuzumab (CLL11) (83, 84); Benda+G, bendamustine+obinutuzumab (GREEN) (85); Ibr+R, ibrutinib+rituximab (Alliance A041202) (86); Benda+Ibr+Ofa, bendamustine+ibrutinib+ofatumumab (CLL2-BIO) (87); Ibr+G, ibrutinib+obinutuzumab (Illuminate PCYC-1130) (88); Benda+Ibr+G, bendamustine+ibrutinib+obinutuzumab (CLL2-BIG) (89); Ibr+FC+G, ibrutinib+fludarabine+cyclophosphamide+obinutuzumab (90); Ven+R (EOCT, End Of Combination Therapy) (EOT, End Of Therapy), venetoclax+rituximab (Murano) (23, 91); Benda+Ven+G, bendamustine+venetoclax+obinutuzumab (CLL2-BAG) (92); Ven+G, venetoclax+obinutuzumab (CLL14) (93); Ven+Ibr (+12 m, months), venetoclax+ibrutinib (TAP Clarity) (94); Ven+Ibr (+18 m, months), venetoclax+ibrutinib (95).