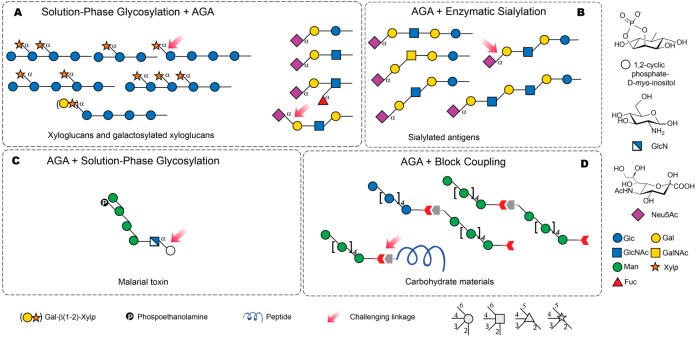
Figure 8.
Selected oligosaccharides assembled using AGA in combination with other techniques. Structures are represented following the SNFG nomenclature.26 The stereochemistry of the glycosidic linkage is β for pyranoses with gluco configuration and α for pyranoses with manno configuration at C2, unless indicated otherwise. Neu5Ac, N-acetylneuramic acid. Linkages that remain a challenge for AGA are indicated by a pink arrow. (A) Solution phase glycosylation is used to install a challenging linkage in a disaccharide that will serve as a building block for AGA. (B) AGA is used to generate a variety of structures that serve as substrates for enzymatic sialylation. (C) AGA provides rapid access to a tetrasaccharide donor, which is then coupled in solution phase to a myo-inositol-containing acceptor. (D) Fragments obtained as AGA are used as scaffolds for the syntheses of carbohydrate materials using block coupling.