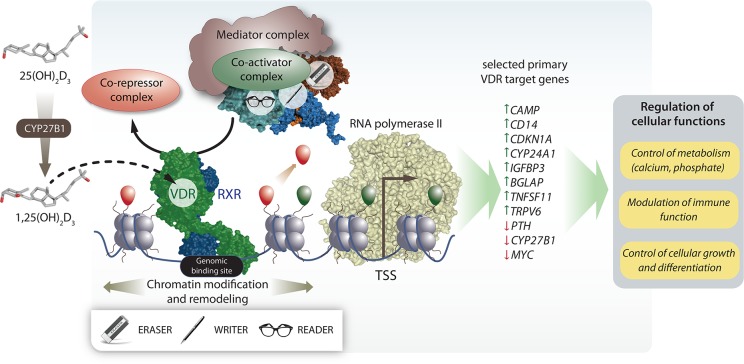
Figure 3.
Vitamin D signaling. 25(OH)D3 is converted by the enzyme CYP27B1 to its biologically most active form 1,25(OH)2D3, which binds to the transcription factor VDR. Upon binding of 1,25(OH)2D3 or synthetic agonists, a conformational change in the LBD is induced leading to cofactor exchanges shifting the balance toward recruitment of coactivator proteins. Co-repressor proteins dissociate from the VDR-RXR heterodimer. In parallel, the mediator complex and chromatin modifying enzymes (readers, writers, and erasers) are recruited in order to handle histone proteins of local nucleosomes around genomic VDR binding sites. In addition, chromatin remodeling complexes are recruited and rearrange nucleosomes at vitamin D-sensitive chromatin regions. Altogether, these chances lead to looping of the distal regulatory elements toward the basal transcriptional machinery with RNA polymerase II and other nuclear adaptor proteins initiating the start of 1,25(OH)2D3-dependent transcription from hundreds to thousands of TSS regions throughout the whole human genome. The ultimate outcome is the increase or decrease of the of primary vitamin D target gene expression followed by changes of indicated cellular functions.