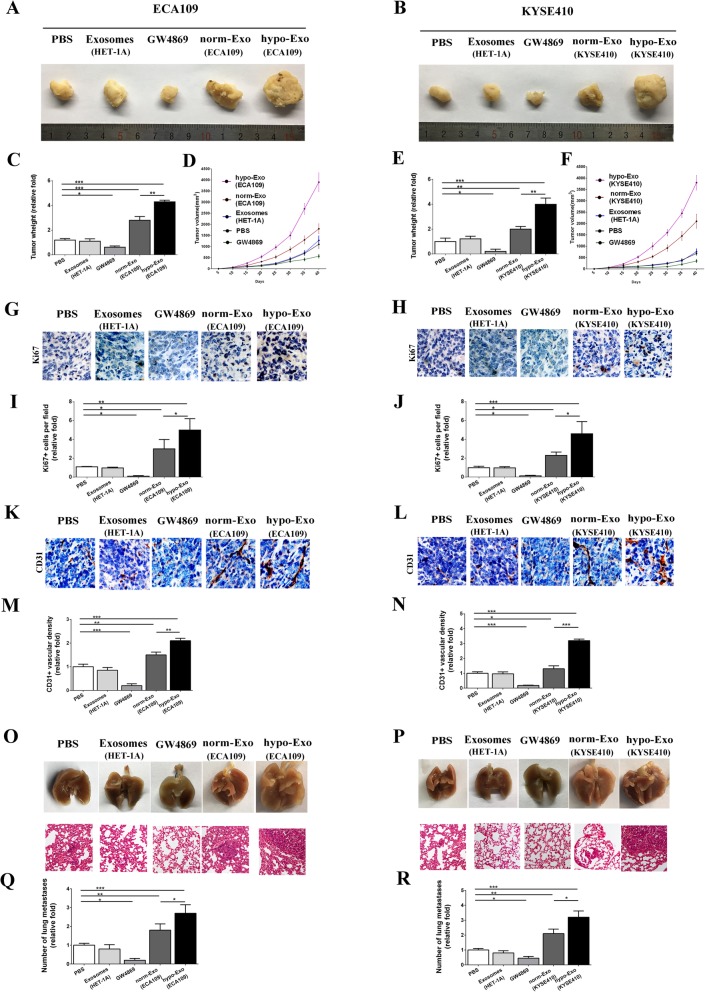
Fig. 5.
Hypoxic exosomes promoted tumor proliferation and angiogenesis in situ and facilitated lung metastasis. Xenograft transplanted tumor models were established through subcutaneously injecting ECA109 (a) or KYSE410 (b) into the nude mice. Then animals were randomly divided into five groups and injected with PBS, 10 μg exosomes from normal squamous esophageal epithelial cell line (HET-1A), exosome release inhibitor (GW4869, 1 mg/kg), 10 μg exosomes from normoxic ECA109 or KYSE410 cells (norm-Exo), or 10 μg exosomes from hypoxic ECA109 or KYSE410 cells (hypo-Exo) every 2 days (a and b). ECA109 or KYSE410 tumor weigh was measured and shown in c and e. Tumor growth curves for ECA109 or KYSE410 tumor models were shown in d and f. Then tumors were analyzed by immunofluorescence microscopy for Ki67 and CD31. Representative pictures of Ki67 were shown in g and h, and quantified for cell proliferation (i and j). Representative pictures of CD31 were shown in k and l, and quantified for vascular density (m and n). Representative images of the general observation the lungs with metastasis nodules and the corresponding H&E images of the tumor edges in lungs (o and p). The metastasis lung nodules were quantified (q and r). Data was presented as mean ± standard deviation (SD). *P < 0.05, **P < 0.01, ***P < 0.001