Abstract
Background
It has been reported that diabetic nephropathy and diabetic retinopathy are associated with each other through a shared pathophysiological mechanism. However, it is quite difficult to differentiate diabetic nephropathy from other glomerular diseases if diabetic retinopathy is absent in patients, and the only way to do this is to perform renal biopsies. The objective of this study was to test the hypothesis that diabetic nephropathy patients with and without diabetic retinopathy have different clinical and laboratory profiles.
Material/Methods
Medical records of type 2 diabetes mellitus patients with confirmed diabetic nephropathy were reviewed and analyzed with appropriate statistical modalities. Presence of arteriolar sclerosis of the carotid artery, abdominal aorta, upper extremities, and first-order aortic branches was regarded as a peripheral vascular disease.
Results
Out of 217 type 2 diabetes mellitus patients with confirmed diabetic nephropathy, retinopathy was present in 106 (48.8%), while 111 (51.2%) had no evidence of retinopathy. About 45% of patients had pure diabetic nephropathy without any diagnosis of non-diabetic renal diseases, of which membranous nephropathy was most common. Diabetic nephropathy patients with retinopathy and those without retinopathy differed in duration of hypertension (p=0.041), serum creatinine (p=0.031), albumin (p=0.001), and erythrocyte sedimentation rate (p=0.001). Moreover, male preponderance (p<0.001), older age (p=0.033), and increased levels of albumin (p=0.033) were significantly associated with pure diabetic nephropathy without retinopathy.
Conclusions
Diabetic nephropathy patients with and without diabetic retinopathy have different clinical and laboratory profiles.
MeSH Keywords: Blood Sedimentation; Creatinine; Diabetes Mellitus, Type 2; Diabetic Nephropathies; Diabetic Retinopathy; Serum Albumin
Background
Diabetes mellitus is a chronic hyperglycemic state characterized by defects in insulin secretion or/and insulin action [1]. The prevalence of diabetes has increased by about 10-fold during the past 3 decades in PR China, which can be attributed to the rapid industrialization and urbanization, increased consumption of high-calorie diet, sedentary lifestyle, and rapid growth of the older population [2]. China has the largest diabetic population in the world, and it is projected to quadruple in size [3]. It has been estimated that about 20–40% of Han Chinese patients with diabetes mellitus have diabetic nephropathy [4]. Diabetic nephropathy is characterized by persistent albuminuria, progressive decline of glomerular filtration rate, and elevation of blood pressure, and is considered a leading cause of end-stage renal disease in PR China, which imposes a considerable burden on the Chinese population and healthcare system [5]. Several investigations have demonstrated the relationship between diabetic retinopathy and diabetic nephropathy among patients with type 2 diabetes mellitus [6,7]. These findings demonstrate the presence of diabetic retinopathy with diabetic nephropathy, but the association is sometimes inconsistent, and diabetic retinopathy and diabetic nephropathy may appear as independent diseases in Han Chinese people [8,9].
Diabetic retinopathy may be helpful in distinguishing the type of kidney pathology in patients with type 2 diabetes mellitus and renal disease [10]. However, if retinopathy is absent in diabetic patients, it is quite difficult to differentiate diabetic nephropathy from other glomerular diseases, and the only way to do this is to perform renal biopsies [7]. Therefore, it is imperative to differentiate the clinical-laboratory characteristics of patients with diabetic nephropathy with and without diabetic retinopathy.
The objective of this analysis was to test the hypothesis that Chinese diabetic patients with confirmed diabetic nephropathy with retinopathy and those without retinopathy have different clinical and laboratory profiles.
Material and Methods
Ethics approval
The study complied with the 2008 Declaration of Helsinki, the strengthening the reporting of observational studies in epidemiology (STROBE) statement, and the law of PR China. The protocol (SU/CL/03/16, dated 24 January 2016) (was approved by the Committee of Ethics on Human Research of The First Hospital of Lanzhou University, China. Since this was an observational retrospective study, waiver of informed consent was granted. The identity of all patients was not disclosed throughout the study, and data were anonymized before randomization.
Inclusion criteria
The records of patients with type 2 diabetes mellitus in VIP Internal Medicine, the First Hospital of Lanzhou University, China from 1 February 2016 to 1 January 2019 and the reference hospitals were evaluated. Patients with diabetic nephropathy confirmed by renal biopsy were considered for further analyses.
Exclusion criteria
Patients without complete details available in the hospital records were excluded from the final analysis. Patients who had negative results of renal biopsy for nephropathy were excluded from the study.
Data collection
All the demographic, clinical, and laboratory data of patients were recorded from the hospital electronic database by using structured data collection forms. The demographic data included age, sex, family history, social parameters, and diabetic complications (hypertension, diabetic vascular disease, peripheral neuropathy, and duration of diabetes). The clinical and laboratory data included the diabetic care profile (glycated hemoglobin (HbA1C), fasting plasma glucose (FPG), and 24-h urinary protein excretion), hemo-analyses and chemistry (hemoglobin, C-reactive protein (CRP), albumin, creatinine, erythrocyte sedimentation rate (ESR)), lipid profile (total cholesterol, triglycerides (TG), high-density lipoproteins (HDL), and low-density lipoprotein (LDL)).
The diagnosis and classification of type 2 diabetes mellitus in the present study were based on the World Health Organization (WHO) criteria (WHO report), which was used as standardized criteria in the hospital. The classification and pathological scoring of diabetic nephropathy were carried out according to the criteria of Tervaert et al. [11]. Moreover, among the study population, diabetic retinopathy was classified according to the international clinical disease severity scale for diabetic retinopathy as no apparent retinopathy, non-proliferative diabetic retinopathy, and proliferative diabetic retinopathy [12]. The Toronto clinical scoring system (score >5) was used to assess peripheral neuropathy [13]. The presence of arteriolar sclerosis of the carotid artery, abdominal aorta, upper extremities, and first-order aortic branches was regarded to as a peripheral vascular disease. Ultrasonography was used to detect the presence of thickening of walls of blood vessels or plaque. Physical activity was categorized using the 6-min walk test. Inability to walk or walking less than 100 m was considered as inactive physical activity. Ability to walk 100–300 m in 6 min was considered as moderate physical activity, and ability to walk 300 m or more in 6 min was considered as physically active.
All the data were screened for accuracy, outliers, and completeness before considering for statistical analysis.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM, Inc., Chicago, IL, USA). Continuous variables are presented as mean with standard deviations (SD). The categorical data were summarized by using frequency with proportion. The Mann-Whitney U test was used to compare the data between continuous variables, while the Fischer exact test was used to differentiate the categorical variables. A value of p<0.05 was considered statistically significant [14].
Results
Medical history of patients
Medical records of 299 type 2 diabetes mellitus patients were reviewed. Among them, 3 patients did not have complete data available in the medical records of the hospitals and 79 patients had negative renal biopsies; therefore, they were excluded from the analysis. A total of 217 patients with diabetic nephropathy confirmed by renal biopsy were included in the study, and among these there was with male preponderance (n=145, 66.8%). The mean age at the time of renal biopsy was 52.11±9.67 years. The prevalence of diabetic neuropathy was 15.7% (n=34/217), while diabetic vascular diseases prevailed in 31.3% (n=68/217) of patients. Almost half (48.8%) of the patients (n=106/217) had diabetic retinopathy. Pure diabetic nephropathy, which is diabetic nephropathy without a confirmed diagnosis of non-diabetic renal diseases, was present in 98 (45.2%) patients, while 115 (54.8%) patients had diabetic nephropathy along with non-diabetic renal diseases. The flow diagram for the analysis is shown in Figure 1.
Figure 1.
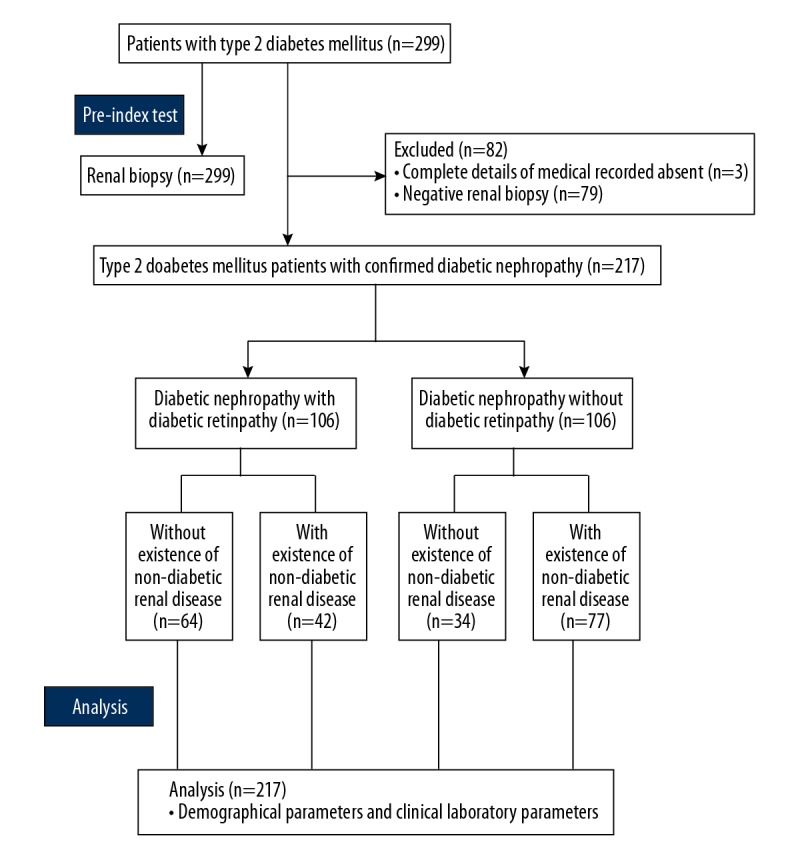
Flow diagram of the analysis.
Prevalence of non-diabetic renal disease along with diabetic nephropathy
The spectrum of non-diabetic renal disease in the selected group of patients included membranous nephropathy, mesangial proliferative glomerulonephritis, membranous proliferative glomerulonephritis, renal tubular interstitial lesion, focal segmental glomerulosclerosis, and the other glomerular disease. Figure 2 illustrates the prevalence of non-diabetic renal disease along with diabetic nephropathy in the study population.
Figure 2.
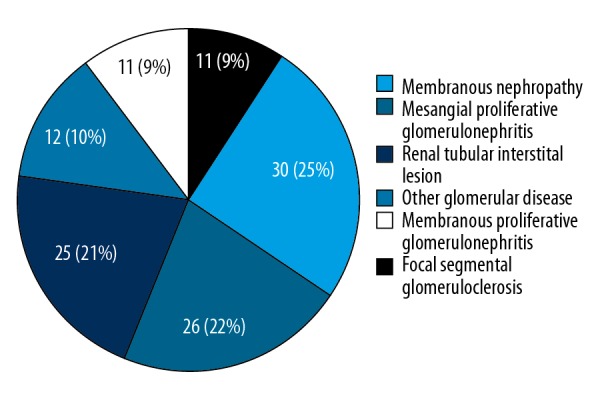
Coexisting non-diabetic renal disease along with diabetic nephropathy in the study population (n=115).
Comparisons between diabetic nephropathy patients with and without diabetic retinopathy
Table 1 compares the demographic characteristics of diabetic nephropathic (pure diabetic nephropathy and diabetic nephropathy with coexisted non-diabetic renal disease) patients with and without diabetic retinopathy. As compared to diabetic nephropathy patients without diabetic retinopathy, the prevalence of diabetic neuropathy (p<0.001) and diabetic vascular diseases (p=0.047) were significantly higher among diabetic nephropathy patients with retinopathy. The duration of hypertension was significantly longer among diabetic nephropathy patients without retinopathy (p=0.041). The other demographics characteristics were equally distributed between diabetic nephropathy patients with and without diabetic retinopathy.
Table 1.
Demographics parameters of diabetic nephropathic patients with or without coexisted non-diabetic renal disease.
| Parameters | Diabetic nephropathic patients with/without coexisting non-diabetic renal disease | ||||
|---|---|---|---|---|---|
| Total | Cohorts | Comparisons between cohorts | |||
| With retinopathy | Without retinopathy | ||||
| Numbers of patients | 217 | 106 | 111 | p-Value | |
| Age | 51.74±8.41 | 49.73±11.31 | 53.12±8.86** | 0.058 | |
| Hight (cm) | 156.15±9.01 | 155.85±8.99 | 155.81±8.71 | 0.974 | |
| Weight (kg) | 58.41±8.22 | 58.11±8.01 | 57.99±7.91 | 0.912 | |
| Body mass index (kg/m2) | 24.45±2.01 | 24.41±1.95 | 24.46±1.81 | 0.845 | |
| Gender | Male | 145 (67) | 72 (68) | 73 (66) | 0.211 |
| Female | 72 (33) | 34 (32) | 38 (34) | 0.062 | |
| Waist circumference (cm) | 86.45±9.41 | 85.88±9.21 | 85.71±9.01 | 0.891 | |
| Hip circumference (cm) | 93.31±9.45 | 93.22±9.31 | 93.18±9.21 | 0.975 | |
| Waist to hip ratio | 0.91±0.09 | 0.90±0.08 | 0.91±0.08 | 0.358 | |
| Waist to height ratio | 0.56±0.02 | 0.55±0.02 | 0.56±0.05 | 0.057 | |
| Diabetic complications | |||||
| Diabetic neuropathy | 34 (16) | 24 (23)* | 10 (9) | <0.001 | |
| Diabetic vascular diseases | 68 (31) | 35 (33)* | 33 (30) | 0.047 | |
| Duration of diabetes (years) | 9.74±7.48 | 10.33±6.77 | 9.14±8.19 | 0.241 | |
| Duration of hypertension (years) | 7.42±7.48 | 6.21±6.77 | 8.54±8.19** | 0.041 | |
| Area of residence | Urban | 99 (46) | 43 (41) | 46 (41) | 0.999 |
| Rural | 118 (54) | 63 (59) | 65 (59) | ||
| Ethnicity | Han Chinese | 199 (92) | 98 (92) | 101 (91) | 0.1 |
| Tibetan | 3 (1) | 3 (3) | 0 (0) | ||
| Mongolian | 15 (7) | 5 (5) | 10 (9) | ||
| Physical activity | Inactive | 49 (23) | 24 (23) | 25 (22) | 0.995 |
| Moderately active | 101 (47) | 49 (46) | 52 (47) | ||
| Active | 67 (30) | 33 (31) | 34 (31) | ||
Continuous data are represented as mean ±SD and constant data are represented as number (percentage). The Mann-Whitney U test was used for continuous data and Fischer exact test for constant data was used for statistical analysis. A p<0.05 was considered significant.
Significantly higher among diabetic nephropathy patients with diabetic retinopathy.
Significantly higher among diabetic nephropathy patients without diabetic retinopathy.
Serum creatinine levels were significantly lower among patients without diabetic retinopathy than among those with diabetic retinopathy (p=0.031). The average albumin levels were significantly different between the 2 groups, with higher levels among diabetic nephropathy patients without diabetic retinopathy (p=0.001). Compared to patients with diabetic retinopathy, those without diabetic retinopathy had significantly lower levels of ESR (39.68±29.90 mm/1 h vs. 64.25±28.62 mm/1 h, p<0.001). The other clinical-laboratory characteristics were equally distributed between diabetic nephropathy patients with and without diabetic retinopathy (Table 2).
Table 2.
Clinical-laboratory characteristics of diabetic nephropathic patients with or without coexisting non-diabetic renal disease.
| Parameters | Diabetic nephropathic patients with/without coexisting non-diabetic renal disease | |||
|---|---|---|---|---|
| Total | Cohorts | Comparisons between cohorts | ||
| With retinopathy | Without retinopathy | |||
| Numbers of patients | 217 | 106 | 111 | p-Value |
| Serum creatinine (uM/L) | 168.52±131.66 | 194.56±112.34* | 133.51±87.66 | 0.031 |
| Albumin (g/L) | 32.34±3.72 | 26.60±3.66 | 34.55±5.31** | 0.001 |
| Hemoglobin (g/L) | 119.79±13.40 | 118.24±17.45 | 121.34±11.14 | 0.072 |
| FPG (mM/L) | 7.15±2.98 | 7.01±3.32 | 7.28±2.92 | 0.581 |
| HbA1C (%) | 6.68±0.98 | 7.01±1.12 | 6.82±1.01 | 0.813 |
| ESR (mm/1 h) | 52.02±27.71 | 64.25±28.62* | 39.68±29.90 | <0.001 |
| CRP (mg/L) | 6.02±3.25 | 5.12±2.81 | 6.98±4.48 | 0.236 |
| Urinary protein (g/24 h) | 6.34±5.01 | 6.75±5.23 | 6.17±6.10 | 0.425 |
| Total CHO (mM/L) | 4.98±2.40 | 4.97±2.25 | 5.36±3.11 | 0.512 |
| TG (mmol/L) | 2.02±1.53 | 1.89±1.31 | 2.26±1.78 | 0.085 |
| HDL (mmol/L) | 0.98±0.33 | 1.00±0.42 | 0.99±0.27 | 0.667 |
| LDL (mmol/L) | 2.64±1.93 | 2.98±2.01 | 3.41±2.40 | 0.376 |
FPG – fasting plasma glucose; HbA1C – glycated hemoglobin; ESR – erythrocytes sedimentation rate; CRP – C-reactive protein; CHO – cholesterol; TG – triglycerides; HDL – high-density lipoproteins; LDL – low-density lipoproteins. Variables are represented as mean ±SD. The Mann-Whitney U test was used for statistical analysis. A p<0.05 was considered significant.
Significantly higher among diabetic nephropathy patients with diabetic retinopathy.
Significantly higher among diabetic nephropathy patients without diabetic retinopathy.
Comparisons between pure diabetic nephropathy patients with and without retinopathy
We compared pure diabetic nephropathy patients (without the existence of non-diabetic renal disease) with and without diabetic retinopathy. Out of 98 patients with pure diabetic nephropathy, 64 (65.3%) patients had diabetic retinopathy, while remaining 34 (34.7%) patients had no evidence of diabetic retinopathy. In the analysis, advanced age (p=0.003) and male sex (p<0.001) were significantly associated with diabetic nephropathy without diabetic retinopathy. More diabetic neuropathy patients had retinopathy (34% vs. 9%), while the other demographic parameters were equally distributed between the 2 groups (Table 3).
Table 3.
Demographics characteristics pure diabetic nephropathic patients.
| Parameters | Diabetic nephropathic patients with/without coexisting non-diabetic renal disease | ||||
|---|---|---|---|---|---|
| Total | Cohorts | Comparisons between cohorts | |||
| With retinopathy | Without retinopathy | ||||
| Numbers of patients | 98 | 64 | 34 | p-Value | |
| Age | 49.46±10.12 | 44.10±9.93 | 54.52±11.76* | 0.033 | |
| Hight (cm) | 157.15±8.15 | 156.45±7.11 | 155.44±7.09 | 0.505 | |
| Weight (kg) | 58.59±8.12 | 57.12±7.12 | 57.02±7.02 | 0.947 | |
| Body mass index (kg/m2) | 24.01±1.12 | 24.24±0.92 | 24.32±0.91 | 0.682 | |
| Gender | Male | 70 (71) | 39 (61) | 31 (91)* | <0.001 |
| Female | 28 (29) | 25 (39) | 03 (9) | 0.088 | |
| Waist circumference (cm) | 86.12±9.13 | 85.11±8.99 | 85.21±7.55 | 0.956 | |
| Hip circumference (cm) | 94.11±10.11 | 93.89±9.15 | 93.25±9.11 | 0.742 | |
| Waist to hip ratio | 0.89±0.11 | 0.90±0.07 | 0.88±0.06 | 0.161 | |
| Waist to height ratio | 0.55±0.02 | 0.56±0.02 | 0.55±0.04 | 0.102 | |
| Diabetic complications | |||||
| Diabetic neuropathy | 25 (26) | 22 (34) | 3 (9) | 0.051 | |
| Diabetic vascular diseases | 37 (38) | 26 (41) | 11 (32) | 0.289 | |
| Duration of diabetes | 9.74±7.48 | 9.13±4.32 | 8.34±4.18 | 0.772 | |
| Duration of hypertension | 7.42±7.47 | 5.13±8.18 | 7.26±6.54 | 0.654 | |
The Mann-Whitney U test was used for continuous data and Fischer exact test for constant data was used for statistical analysis. A p<0.05 was considered significant.
Significantly higher among diabetic nephropathy patients without diabetic retinopathy.
Laboratory profiles showed that only the levels of albumin were significantly higher among diabetic nephropathy patients without diabetic retinopathy as compared to diabetic nephropathy patients with diabetic retinopathy (p=0.033), while the other laboratory parameters were equally distributed between the 2 groups (Table 4).
Table 4.
Clinical-laboratory characteristics of pure diabetic nephropathic patients.
| Parameters | Diabetic nephropathic patients without non-diabetic renal disease | |||
|---|---|---|---|---|
| Total | Cohorts | Comparisons between cohorts | ||
| With retinopathy | Without retinopathy | |||
| Numbers of patients | 98 | 64 | 34 | p-Value |
| Serum creatinine (uM/L) | 168.52±121.87 | 198.68±149.56 | 182.67±132.56 | 0.548 |
| Albumin (g/L) | 30.78±6.92 | 28.42±7.11 | 32.86±7.01* | 0.033 |
| Hemoglobin (g/L) | 117.52±16.61 | 110.75±16.55 | 115.44±20.33 | 0.301 |
| FPG (mM/L) | 6.98±2.44 | 6.18±2.11 | 6.99±2.86 | 0.060 |
| HbA1C (%) | 6.99±1.08 | 6.89±2.11 | 7.01±1.36 | 0.550 |
| ESR (mm/1 h) | 53.22±30.93 | 63.54±30.05 | 43.78±30.56 | 0.136 |
| CRP (mg/L) | 5.94±3.51 | 5.10±2.89 | 5.89±5.15 | 0.619 |
| Urinary protein (g/24 h) | 5.82±4.34 | 6.88±5.13 | 5.94±3.98 | 0.884 |
| Total CHO (mM/L) | 5.01±1.98 | 5.12±2.10 | 4.92±1.34 | 0.702 |
| TG (mmol/L) | 2.16±1.48 | 1.78±0.97 | 2.51±1.90 | 0.261 |
| HDL (mmol/L) | 0.89±0.42 | 1.11±0.50 | 0.84±0.43 | 0.432 |
| LDL (mmol/L) | 2.64±1.93 | 2.99±1.51 | 3.26±1.13 | 0.872 |
FPG – fasting plasma glucose; HbA1C – glycated hemoglobin; ESR – erythrocytes sedimentation rate; CRP – C-reactive protein; CHO – cholesterol; TG – triglycerides; HDL – high-density lipoproteins; LDL – low-density lipoproteins. Variables are represented as mean ±SD. The Mann–Whitney U test was used for statistical analysis. A p<0.05 was considered significant.
Significantly higher among diabetic nephropathy patients without diabetic retinopathy.
Discussion
We investigated the demographic and clinical-laboratory features of diabetic nephropathy patients and focused on parameters differentiating between diabetic nephropathy with retinopathy and diabetic nephropathy without retinopathy. Although it has been well documented that diabetic nephropathy and diabetic retinopathy have similar pathological mechanisms, research results also suggest differences between diabetic nephropathy and diabetic retinopathy; for example, severe diabetic retinopathy can occur in the absence of diabetic nephropathy [15]. Also, overt diabetic nephropathy without diabetic retinopathy is common in Korean populations [16]. The genetic similarity between Korean populations and Han Chinese populations is more than 99% [17]. Few studies have indicated no relationship between diabetic nephropathy and diabetic retinopathy, and the prevalence of fundus lesions is inconsist across these studies [15,18]. Furthermore, the clinical and laboratory characteristics of patients with diabetic nephropathy without diabetic retinopathy are not fully understood. In this context, the present study will be a significant addition to the existing literature on diabetic nephropathy.
The present study is a detailed comparison of diabetic nephropathic patients, either with pure diabetic nephropathy or diabetic nephropathy with coexisting non-diabetic renal disease, with and without diabetic retinopathy. When cases of diabetic nephropathy (including both pure diabetic nephropathy and diabetic nephropathy with coexisting non-diabetic renal disease) were evaluated, 7 parameters significantly differed between the 2 groups. The prevalence of diabetic neuropathy was higher among diabetic nephropathic patients with retinopathy as compared to diabetic nephropathic patients without retinopathy. These results are consistent with the findings of previous investigations demonstrating the association of peripheral neuropathy with diabetic retinopathy [9,19]. Diabetic vascular disease was found to be associated with retinopathy in the present study. However, the findings of a retrospective observational study in a Chinese population did not show any such association [9]. The levels of serum creatinine were significantly higher among patients with diabetic retinopathy, which explicitly indicate the higher prevalence of diabetic complications among patients with retinopathy. The levels of albumin were significantly lower among patients with diabetic retinopathy. The longer duration of diabetes among diabetic retinopathy patients suggests that chronic metabolic processes cause the decomposition of albumin, resulting in lower levels in the diabetic retinopathy cohort [9]. Other parameters differing between the 2 groups were the duration of hypertension and ESR, which were significantly longer among patients with diabetic nephropathy and diabetic retinopathy. Diabetic nephropathy and diabetic retinopathy are different diseases.
Among patients with proteinuria, in the absence of diabetic retinopathy, it is sometimes difficult to differentiate between diabetic nephropathy and membranous nephropathy, particularly when facilities for renal biopsies are unavailable or when a renal biopsy is not suitable due to bleeding tendencies. In this context, we performed the present analysis comparing pure diabetic nephropathy and membranous nephropathy without retinopathy to find some clinical clues for differential diagnoses. The subgroup analysis revealed that patients with membranous nephropathy had higher levels of HDL and cholesterol, which are the cardinal features of membranous nephropathy. However, other parameters were equally distributed between the 2 groups. These findings are consistent with other retrospective observational analyses[9]. The results of our analysis show that lipid profile should be considered in differentiating between diabetic nephropathy and diabetic retinopathy.
The analysis also evaluated the impact of diabetic retinopathy among patients with pure diabetic nephropathy. The findings showed that patients with diabetic nephropathy but without retinopathy were significantly associated with old age and the male preponderance in a group of patients having diabetic nephropathy without retinopathy. These results agree with a retrospective study in a Chinese population [9], but disagree with the results of a cross-sectional community-based study [19]. These disagreements may be due to the fact that the cross-sectional study assessed an Indo-Aryan population and the present observational study assessed a Chinese population. Our study indicates that Chinese diabetic nephropathic patients without retinopathy are more likely to be older males with higher levels of albumin.
The analysis demonstrated no association of hypertension and its duration with the development of diabetic macular edema (DME). Our results are consistent with those of a Taiwanese population-based cohort study [8]. Moreover, the results of the present study showed a higher proportion of younger patients having diabetic nephropathy with retinopathy. These findings also agree with the results of a Taiwanese population-based cohort study [8], which reported that young patients with diabetic nephropathy had higher risk of DME development. Further controlled studies are needed to define the relationship of demographics with the development and prognosis of diabetic nephropathy and diabetic retinopathy in Han Chinese people.
The current analysis was limited by its small sample size and selection bias. Since renal biopsy was performed in a small group of patients, it is quite possible that the inclusion of patients who did not undergo renal biopsy during the study may have affected the results. However, our study is one of a few reports in a Chinese population differentiating the characteristics of diabetic nephropathy patients with or without diabetic retinopathy.
Conclusions
This study demonstrates that diabetic nephropathy patients with and without diabetic retinopathy present with different clinical and laboratory profiles. Those with diabetic nephropathy without diabetic retinopathy tended to have less renal damage, fewer diabetic complications, and better prognosis as compared to patients with diabetic nephropathy with diabetic retinopathy. We found several demographic, clinical, and laboratory characteristics differentiating the 2 groups of patients. These parameters should be identified as early as possible when a renal biopsy is unavailable or unwarranted. Large-scale, well-controlled cohort studies are needed to confirm these findings and to determine effective indicators of diabetic nephropathy and non-diabetic renal diseases.
Acknowledgments
Authors thank the medical and non-medical staff of West China School of Public Health, Chengdu, Sichuan, China, Sichuan University, Sichuan, China, and the First Hospital of Lanzhou University, Lanzhou, Gansu, China.
Abbreviations
- STROBE
the reporting of observational studies in epidemiology
- HbA1C
glycated hemoglobin
- FPG
fasting plasma glucose
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- TG
triglycerides
- HDL
high-density lipoproteins
- LDL
low-density lipoprotein
- WHO
World Health Organization
- SD
standard deviations
- DME
diabetic macular edema
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Kerner W, Bruckel J German Diabetes Association. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(7):384–86. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Weng JP, Bi Y. Epidemiological status of chronic diabetic complications in China. Chin Med J (Engl) 2015;128(24):3267–69. doi: 10.4103/0366-6999.171350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Xu Z, Han P, Li T. Glycated albumin and ratio of glycated albumin to glycated hemoglobin are good indicators of diabetic nephropathy in type 2 diabetes mellitus. Diabetes Metab Res Rev. 2017;33(2) doi: 10.1002/dmrr.2843. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z, Liu N, Wang F. Epigenetic regulations in diabetic nephropathy. J Diabetes Res. 2017;2017 doi: 10.1155/2017/7805058. 7805058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Rubeaan K, Youssef AM, Subhani SN, et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: A Saudi National Diabetes Registry-based study. PLoS One. 2014;9(2):e0088956. doi: 10.1371/journal.pone.0088956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He F, Xia X, Wu XF, et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: A meta-analysis. Diabetologia. 2013;56(3):457–66. doi: 10.1007/s00125-012-2796-6. [DOI] [PubMed] [Google Scholar]
- 8.Jeng CJ, Hsieh YT, Yang CM, et al. Diabetic retinopathy in patients with diabetic nephropathy: Development and progression. PLoS One. 2016;11(8):e0161897. doi: 10.1371/journal.pone.0161897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XQ, Zheng X, Chen M, Zhao MH. Characteristics of diabetic nephropathy patients without diabetic retinopathy: A retrospective observational study. Medicine. 2017;96(18):e6805. doi: 10.1097/MD.0000000000006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J Diabetes. 2013;4(6):245–55. doi: 10.4239/wjd.v4.i6.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tervaert TW, Mooyaart AL, Amann K, et al. Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4(6):290–94. doi: 10.4239/wjd.v4.i6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Mao JP, Yan X. [Toronto clinical scoring system in diabetic peripheral neuropathy]. J Cent South Univ (Med Sci) 2008;33(12):1137–41. [in Chinese] [PubMed] [Google Scholar]
- 14.Katulanda P, Constantine GR, Mahesh JG, et al. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka – Sri Lanka diabetes, Cardiovascular Study (SLDCS) Diabet Med. 2008;25(9):1062–69. doi: 10.1111/j.1464-5491.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 15.Kotlarsky P, Bolotin A, Dorfman K, et al. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int Ophthalmol. 2015;35(1):59–66. doi: 10.1007/s10792-014-0018-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee WJ, Sobrin L, Lee MJ, et al. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3) Invest Ophthalmol Vis Sci. 2014;55(10):6547–53. doi: 10.1167/iovs.14-15001. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Lu D, Chung YJ, Xu S. Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations. Hereditas. 2018;155:19. doi: 10.1186/s41065-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Chen X, Xie Y, et al. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23(6):1940–45. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 19.Katulanda P, Ranasinghe P, Jayawardena R. Prevalence of retinopathy among adults with self-reported diabetes mellitus: The Sri Lanka diabetes and cardiovascular study. BMC Ophthalmol. 2014;14:100. doi: 10.1186/1471-2415-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]


