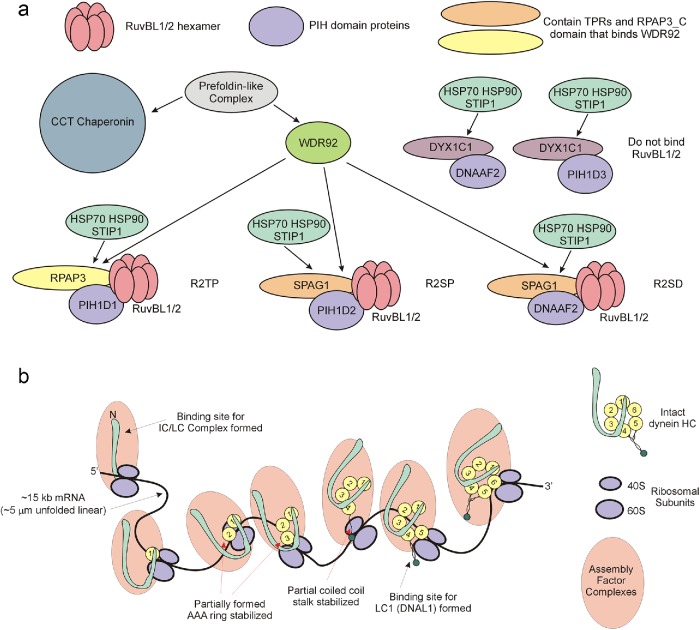
FIGURE 8:
The role of WDR92 in the axonemal dynein assembly pathway. (a) Diagram illustrating the central role that WDR92 may play in coordinating association of R2TP (and variant) complexes with the prefoldins and the CCT chaperonin. WDR92 interacts directly with both prefoldins and the RPAP3_C domains present in RPAP3 and SPAG1. These in turn scaffold interactions with the RuvBL1/2 AAA+ ATPases, and PIH proteins that mediate recruitment of HSP70 and HSP90. As eukaryotes (such as yeast, higher plants, and nematodes) missing axonemal dyneins also lack WDR92, how prefoldins and chaperonins associate with R2TP in these organisms remains unclear. Furthermore, although clearly essential for axonemal dynein HC formation, why cytoplasmic and IFT dynein HCs, which have similar folding requirements, are unaffected remains unexplored. (b) Model illustrating the synthesis of dynein HCs. Each spliced HC mRNA is ∼15 kb, which equates to an unfolded linear molecule of ∼5 μm; at an incorporation rate of 5 residues/s, a ribosome would take ∼13–15 min to track along an mRNA of this length. Simultaneous association of numerous ribosomes would lead to a group of partially synthesized dynein HCs associated with a complex array of cytoplasmic assembly and stabilization factors. Given the circular arrangement of AAA domains within the dynein motor unit, an HC can be fully stabilized only once the terminal AAA unit has been synthesized. However, binding sites for individual dynein components such as LC1 (DNAL1), which associates near the ATP-dependent microtubule-binding domain, or the IC/LC complex, which interacts with the N-terminal region, may become available and occupied before completion of HC synthesis.