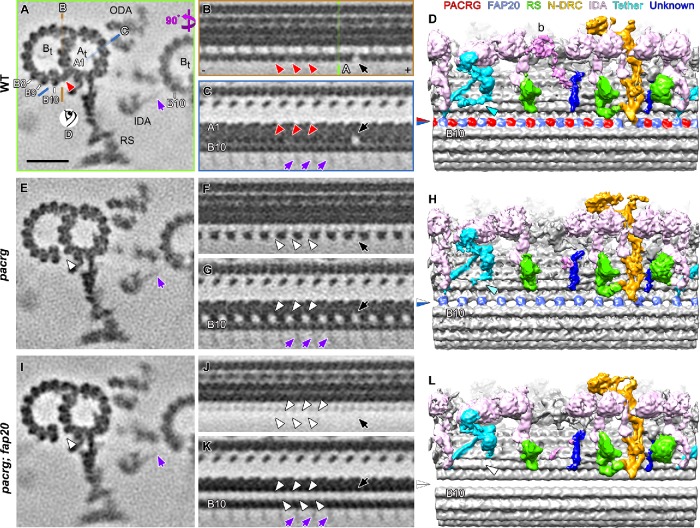
FIGURE 4:
Cryo-ET of the Chlamydomonas pacrg and pacrg; fap20 mutants reveals structural defects in the IJ of the DMTs. (A–L) Subtomogram averages of the axonemal repeats from WT (A–D), the pacrg mutant (E–H), and the pacrg; fap20 double mutant are shown as cross-sectional (A, E, I; 3 nm thick) and two longitudinal (B, C, F, G, J, K; 2 nm thick) tomographic slices and as longitudinal three-dimensional isosurface renderings (D, H, L). The locations of the tomographic slices shown in B and C (orange and blue lines) and the viewing direction of D (eye symbol) are indicated in A, whereas the location of slice shown in A is indicated in B (green line). Densities that were present in WT axonemes but missing from pacrg and pacrg; fap20 are highlighted by red and white arrowheads, respectively. The blue/red arrowhead in D indicates the WT IJ consisting of repeating PACRG/FAP20 heterodimers, which was missing the PACRG subunit in the pacrg mutant (blue/white arrowhead in H) or the entire IJ density in pacrg;fap20 mutant (white/white arrowhead in L). Note that the I1 tether base structure (Fu et al., 2018) present in WT (cyan arrowhead in D) is reduced in pacrg (H) and missing in pacrg; fap20 (white arrowhead in L). The protofilaments (A1, B8–10) are numbered according to the prevailing numbering system (Linck and Stephens, 2007). Other labels: At, A-tubule; Bt, B-tubule; N-DRC, nexin-dynein regulatory complex; RS, radial spoke; ODA, outer dynein arm; + and − end, microtubule polarity; black arrow, IJ hole. Note the densities of the stalk and microtubule-binding domains of the inner dynein arms (IDAs) from the neighboring DMT bound to protofilament B10 (purple arrows in A, C, E, G, I, K). These densities are weak and exhibit an 8-nm periodicity, because 1) dyneins on the neighboring DMT n-1 bind to a specific site on the tubulin dimers on the DMT n, but 2) the position of axonemal repeats on the DMT n that are averaged vary among the nine DMTs of a flagellum. Scale bar: 20 nm (A, valid for all tomographic slices).