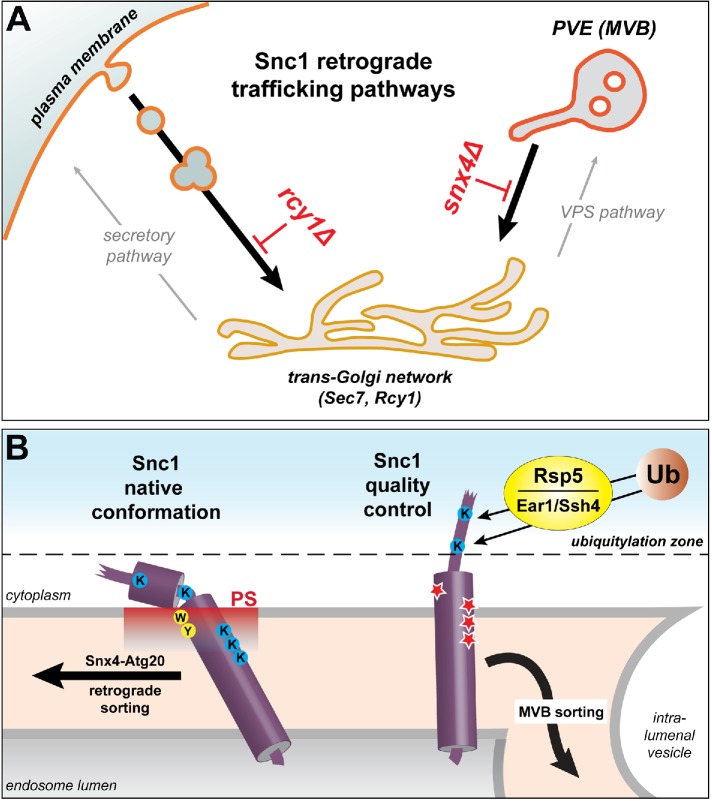
FIGURE 7:
Model of Snc1 trafficking pathways and structural features of Snc1 required for Snx4-dependent trafficking. (A) Snc1 retrograde trafficking pathways in the endo-vacuolar system. Anterograde Snc1 trafficking bifurcates at the TGN, where it can be directed to the plasma membrane via the secretory pathway or the endo-vacuolar system via the VPS pathway. On endocytosis of Snc1 from the plasma membrane, Rcy1 is required for its entry into the TGN, a compartment in which both Sec7 and Rcy1 reside. Retrieval from the PVE to the TGN is mediated by Snx4-Atg20. (B) Structural features of Snc1 that play vital roles in Snx4-dependent trafficking of native Snc1 and sorting of mutant Snc1 proteins into the degradative MVB pathway. In wild-type Snc1, aromatic residues (yellow) anchor into the membrane bilayer, forcing the transmembrane domain to tilt (Kweon et al., 2003; Bowen and Brunger, 2006), allowing downstream basic lysine residues (blue) to snorkel and interact with the membrane surface. This orientation of the transmembrane domain allows proper display of the Snx4-Atg20–dependent sorting signal located in the JMH. We propose that lysine residues within this region and those in the transmembrane segment that snorkel to the membrane surface promote Snc1 residence in PS-rich regions (red) of the endosome membrane, which facilitates sorting into the Snx4-Atg20 retrograde pathway (Ma et al., 2018). Mutant Snc1-DDD and Snc1-W86R (Lewis et al., 2000) (indicated in red stars) are subjected to protein quality control by sorting into the degradative MVB pathway, requiring ubiquitylation of Rsp5, working with the Ear1 and/or Ssh4 adapters. We propose that these mutations cause unfolding of the juxtamembrane and the ‟top” of the transmembrane helices, allowing access of Lys75 and Lys83 to the “ubiquitylation” zone defined by Sardana et al. (2019).