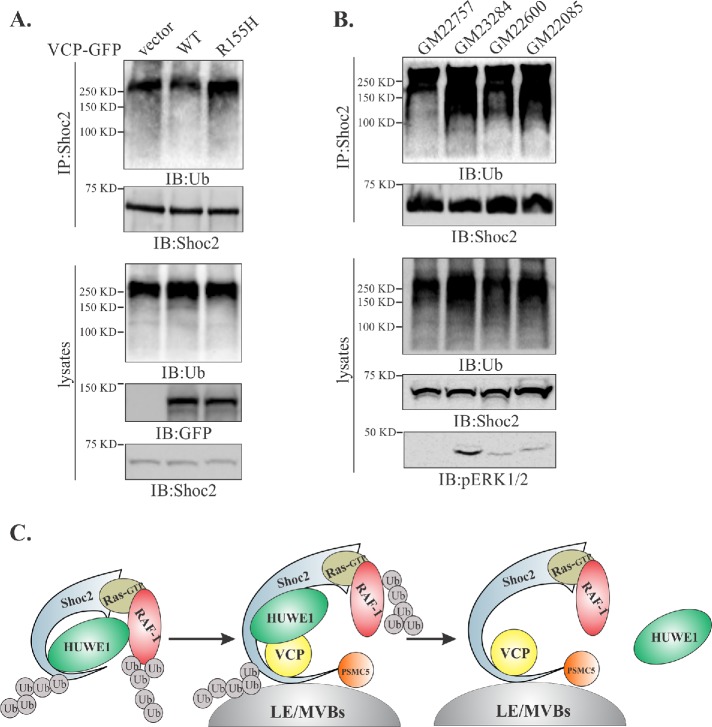
FIGURE 5:
Ubiquitination of Shoc2 and RAF-1 is altered in fibroblasts from IBMPFD patients. (A) Endogenous Shoc2 was precipitated from the denatured cell lysates of Cos1 cells expressing WT or R155H mutant of VCP-GFP. Shoc2 ubiquitination was detected with anti-Ub antibody. (B) Endogenous Shoc2 was immunoprecipitated from the denatured lysates of primary fibroblast using anti-Shoc2 antibodies. GM23284, GM22600, and GM22085—fibroblasts harboring VCP R155H mutation; GM22757—normal relative control. Shoc2 ubiquitination was detected with anti-Ub antibody. pERK1/2 was analyzed using specific pERK1/2 (p42/44) antibodies. The results in each panel are representative of those from three independent experiments. (C) Working model depicting what is currently understood for the mechanisms modulating the Shoc2-mediated ERK1/2 signals. Activation of the ERK1/2 pathway is followed by HUWE1-mediated ubiquitination of Shoc2 and RAF-1 causing the adjustments in the amplitude of ERK1/2 signaling. Thereafter, PSMC5 facilitates the recruitment of the Shoc2 complex to endosomes where VCP recognizes ubiquitinated Shoc2 and HUWE1 and sequesters or “segregates” HUWE1 from the Shoc2 complex, possibly aiding in the “reactivation” of the Shoc2 signaling module.