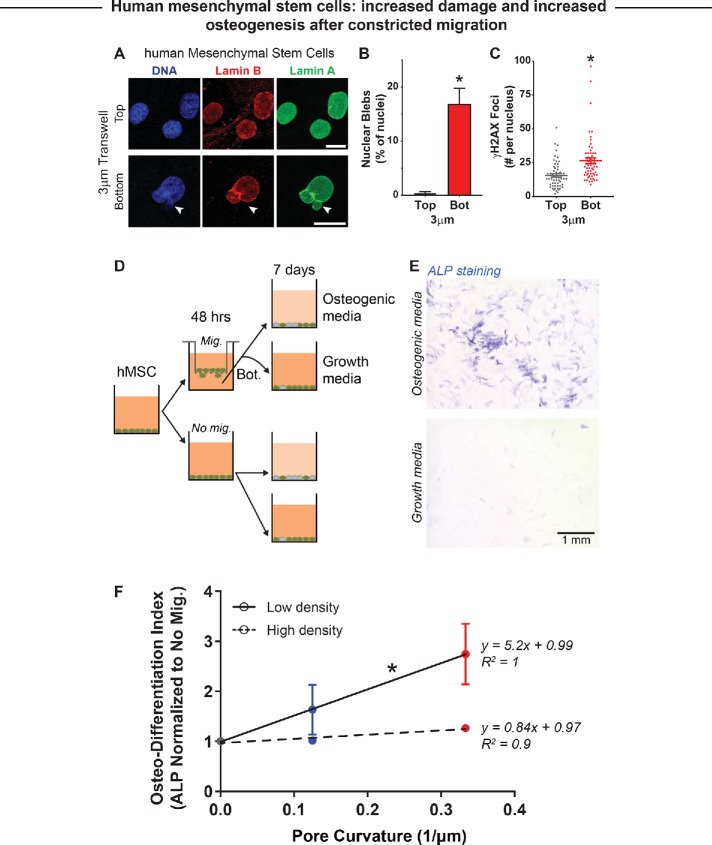
FIGURE 7:
Constricted migration of human mesenchymal stem cells (hMSC) enhances osteogenic differentiation. (A) Representative images of nonmigrated and migrated hMSCs on the top and bottom, respectively, of a 3 µm–pore transwell membrane. White arrow indicates a nuclear bleb formed after 3 µm–pore migration. Scale bar = 10 µm. (B) Migration of hMSCs through 3-µm pores causes a significant increase in nuclear blebbing. (C) Constricted migration of hMSCs also leads to a significant increase in DNA damage, as measured by γH2AX foci. N = 46–61 cells. * indicates significant (p < 0.05) difference from the no-migration condition. (D) Bone marrow–derived hMSCs are either cultured in monolayer (No mig.) or subjected to 3- and 8-µm transwell migration (Mig.); the nonmigrated and migrated cells are then subjected to either growth media or osteogenic media to induce osteogenic differentiation. (E) Level of osteogenic differentiation was measured by alkaline phosphatase (ALP) staining with Fast Blue RR salt (Sigma). Increased ALP activity is a well-known marker for osteogenic differentiation, as shown by the blue staining. Scale bar = 1 mm. (F) Increase in ALP activity is highest for cells that migrated through 3-µm constrictions and were then cultured at low seeding density (low = 10,000 cells/cm2; high = 20,000 cells/cm2). This is a combination of suppressed spontaneous osteogenic differentiation and enhanced induced differentiation. High seeding density seems to suppress the migration-induced enhancement of osteogenic differentiation. N = 9057–16,814 cells. * indicates significant difference between the slope coefficients of low and high seeding density (p < 0.05).