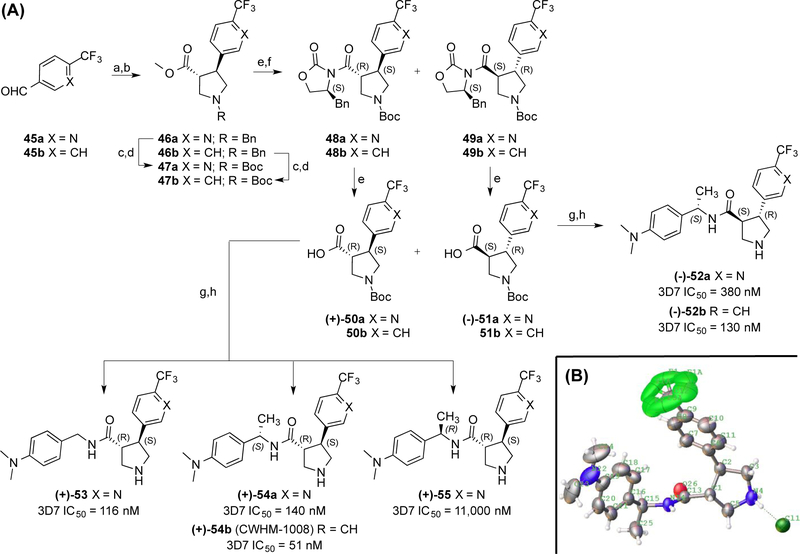
Figure 5. Synthesis and SAR of pyrrolidine enantiomers 52–55.
(A) IC50 values are given as average potency values in the Pf 3D7 assay (n≥3). Reagents and conditions: (a) Ph3P=CHCO2Me; (b) TFA, Me3SiCH2N(Bn)CH2OMe; (c) NH4CO2H, Pd/C; (d) Et3N, Boc2O; (e) LiOH; (f) (S)-4-benzyl-2-oxazolidinone, pivaloyl chloride, TEA, LiCl; (g) ArCH2NH2, HATU; (h) HCl. (B) ORTEP representation of the crystal structure of 54b as an HCl salt.