Abstract
Background
Mortality is higher in patients with chronic kidney disease (CKD) than in the general population, but little information is available on CKD-related mortality that is representative of the Korean population. Our objective was to investigate mortality risk in Korean patients with CKD.
Methods
We identified patients with incident CKD who had not undergone dialysis or kidney transplantation between January 1, 2003 and December 31, 2007 in Korea using the database of the Korean National Health Insurance Service-National Sample Cohort, and stratified the population into the following three groups: group 1 (n = 1,473), controls; group 2 (n = 2,212), patients with diabetes or hypertension, but without CKD; and group 3 (n = 2,212), patients with CKD. We then monitored them for all-cause mortality until December 2013.
Results
A total of 1,473 patients were included in this analysis. During the follow-up period, 941 patients in group 3 died (134 deaths/1,000 person-years) compared with 550 deaths in the group 2 (34 deaths/1,000 person-years) and 459 deaths in group 1 (30 deaths/1,000 person-years). The rate ratio for mortality rate was 4.5, and the hazard ratio for mortality was 4.88 (95% confidence interval [CI], 4.36–5.47, P < 0.001) in patients in group 3 compared with age- and sex-matched controls (group 1). The rate ratio for mortality rate was 4.0, and the hazard ratio for mortality was 4.36 (95% CI, 3.92–4.85, P < 0.001) in patients in group 3 compared with patients in group 2.
Conclusion
In this nationally representative sample cohort, excess mortality was observed in Korean patients with incident CKD.
Keywords: Diabetes mellitus, Hypertension, Korea, Mortality, Renal insufficiency, chronic
Introduction
Chronic kidney disease (CKD) is a global public health problem, and its prevalence has dramatically increased with the aging population and their chronic diseases. A recent study reported that the global prevalence of CKD increased by 87% and the death rate from CKD rose by 98% from 1990 to 2016 [1]. In Korea, 5% of the population had decreased kidney function, specifically a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 in 2009; the prevalence of CKD was 23% in people in their 60s and greater than 35% in those 70 years and older [2].
Patients with CKD have a higher mortality rate compared to the general population [3]. However, most studies of CKD have focused on patients undergoing chronic dialysis, and to our knowledge there are no studies that have investigated mortality among pre-dialytic patients with incident CKD in Korea. Moreover, most studies of CKD have concentrated on the progression of end-stage renal disease (ESRD), even though many CKD patients die before ESRD progression [4]. Keith et al [5] showed that patients with CKD were ten times more likely to die than to progress to ESRD, and even a mild decrease in renal function was associated with a significantly increased risk of mortality. Unfortunately, there have been no studies comparing mortality in patients with CKD to that in the healthy population in Korea. Therefore, we investigated the mortality rate in Korean patients with CKD compared with healthy controls and in patients with diabetes or hypertension using the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC).
Methods
Data
The investigation was performed using data from the NHIS-NSC of Korea. The cohort was composed of 2.2% of the total eligible Korean population (baseline population = 1,025,340 people), which was selected as a representative sample in 2002 using systematic stratified random sampling. The data contain information on demographics such as age group (< 1 year, 1 to 4 years, 5-year age groups between 5 and 84 years, and ≥ 85 years), sex, income level, healthcare utilization, prescriptions, and diagnostic codes based on the International Classification of Diseases, 10th Revision (ICD-10). Detailed information about the NHIS- NSC can be found elsewhere [6]. This study was approved by the Institutional Review Board (IRB) of the Ewha Womans University Mokdong Hospital (IRB number: EUMC 2018-09-007). The requirement for informed consent from patients was waived due to the retrospective design of the study.
Study sample
We defined pre-dialytic CKD as those who had new insurance claims with the diagnostic codes of ‘N18.x’ (CKD; N18, N18.1, N18.2, N18.3, N18.4, N18.5, and N18.9) from January 2003 to December 2007. We then excluded participants who had claims with the ‘N18.x’ code between January and December 2002 in order to enroll only incident cases. Of those, anyone diagnosed with ESRD at study enrollment was excluded, and ESRD was identified when subjects had insurance claims with a regular dialysis (hemodialysis and/or peritoneal dialysis) treatment code (O7010, O7020, and O7070). Additionally, subjects who had undergone kidney transplantation were also excluded at enrollment and throughout the study period (R3280).
In addition, we stratified the subjects into following three groups: group 1, controls; group 2, patients with diabetes or hypertension, but without CKD; and group 3 patients with CKD. We selected age- and sex-matched participants with a Charlson comorbidity index (CCI) of 0 [7] without ‘N18.x’ from January 2002 to December 2007 to construct the healthy control group (group 1). Group 1 and group 2 were individually matched to the CKD patients according to age and sex at a 1.5:1.5:1 ratio. Finally, 1,473 patients were enrolled in group 3, and 2,212 patients in groups 2 and 3, respectively.
Study outcomes
All enrolled participants were monitored for all-cause mortality during the study follow-up period. Information related to deaths was provided from the Korea National Statistical Office with follow-up data from 2002 to 2013 available.
Statistical analysis
Patients were divided into age groups (< 1 year, 1 to 4 years, 5-year age groups; < 1, 1–4, every 5-year interval from 5–84, and ≥ 85 years), and matching was carried out in a ten-year unit to select as many comparators as possible in the same age group. However, when analyzed, they were reclassified based on age (younger than 65 years old vs. 65 years old or older) and sex. The crude incidence rates were calculated by dividing the number of subjects with a given event by person-years, which were expressed as cases per 1,000 person-years. In addition, Kaplan–Meier analysis was conducted to compare the mortality rate between the CKD group and the control group with log-rank tests. Cox proportional hazards analysis was also performed to examine time-to-event association with all-cause mortality. We adjusted for age and sex using a multivariate model. This analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Among 1,025,340 randomly selected individuals from the NHIS-NSC, which is representative of the whole Korean population, 1,473 patients (864 males and 609 females) who developed CKD between January 1, 2003 and December 31, 2007 were enrolled in this study. The characteristics of the participants stratified into the three groups are summarized in Table 1. Approximately 58% of patients across all groups were older than 65 years old, and the proportion of males was higher overall (59% males vs. 41% females).
Table 1.
Patient characteristics by group
| Characteristic | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Total | 2,212 (100) | 2,212 (100) | 1,473 (100) |
| Age (yr) | |||
| < 65 | |||
| Male | 592 (26.8) | 592 (26.8) | 392 (26.6) |
| Female | 344 (15.6) | 344 (15.6) | 234 (15.9) |
| ≥ 65 | |||
| Male | 710 (32.1) | 710 (32.1) | 472 (32.0) |
| Female | 566 (25.6) | 566 (25.6) | 375 (25.5) |
| Annual incidence (year) | |||
| 2003 | 368 (16.6) | 368 (16.6) | 251 (17.0) |
| 2004 | 373 (16.9) | 373 (16.9) | 247 (16.8) |
| 2005 | 450 (20.3) | 450 (20.3) | 298 (20.2) |
| 2006 | 483 (21.8) | 483 (21.8) | 320 (21.7) |
| 2007 | 538 (24.3) | 538 (24.3) | 357 (24.2) |
| Comorbidities | |||
| Hypertension | NC | 1,983 (89.7) | 1,335 (90.6) |
| Diabetes mellitus | NC | 641 (29.0) | 825 (56.0) |
| Cardiovascular disease | NC | 188 (8.5) | 386 (26.2) |
| Malignancy | NC | 149 (6.7) | 257 (17.4) |
Data are presented as number (%). The sum of the percentages does not equal 100% because of rounding.
Group 1, control; group 2, patients with diabetes or hypertension but without chronic kidney disease (CKD); group 3, patients with CKD.
NC, not collected.
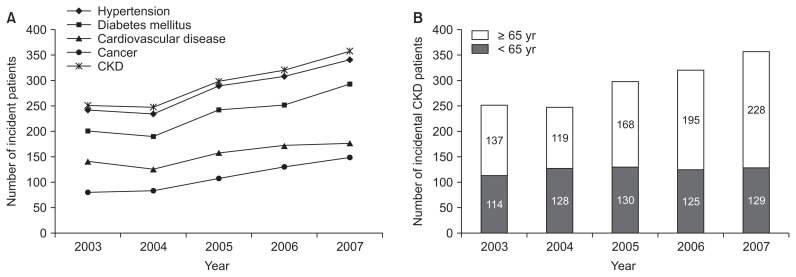
The number of patients who developed CKD showed an increasing trend over time (Fig. 1). Regarding selected comorbidities identified by codes in group 3, more than 90.6% had hypertension, 26.2% had cardiovascular disease, 56.0% had diabetes mellitus and 17.5% had cancer at enrollment.
Figure 1. Annual trends in incidence of (A) diseases and (B) chronic kidney disease (CKD).
Impact of CKD on mortality compared with controls
Over the study period, 941 (63.9%) of the participants with CKD died, compared with 550 (24.9%) of the participants in group 2 and 459 (20.8%) of the participants in group 1. The death rate was higher in the CKD group than that in the other two groups (Table 2, 3, Fig. 2). The crude death rate among individuals with CKD was 134 per 1,000 person-years compared with 30 per 1,000 person-years in group 1 and 34 per 1,000 person-years in group 2; the rate ratio for mortality was 4.0 (group 3 vs. group 2) and 4.5 (group 3 vs. group 1) (Table 2, 3). When stratified by age with a reference of 65 years old, the crude mortality rate was higher in participants older than 65 years compared with those younger than 65 years in all groups.
Table 2.
Mortality rate ratio of group 3 compared to group 1
| Group 1 | Group 3 | Mortality rate | Rate ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Observed deaths (n) | Person-years | Observed deaths (n) | Person-years | Group 1 | Group 3 | ||||
| Total | 459 | 15,312 | 941 | 6,998 | 30 | 134 | 4.5 | ||
| Age (yr) | < 65 | Male | 23 | 4,485 | 165 | 2,375 | 5 | 69 | 13.5 |
| Female | 7 | 2,740 | 83 | 1,552 | 3 | 53 | 20.9 | ||
| ≥ 65 | Male | 249 | 4,402 | 383 | 1,697 | 57 | 226 | 4.0 | |
| Female | 180 | 3,684 | 310 | 1,374 | 49 | 226 | 4.6 | ||
Group 1, control; group 3, patients with chronic kidney disease.
Table 3.
Mortality rate ratio of group 3 compared to group 2
| Group 2 | Group 3 | Mortality rate | Rate ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Observed deaths (n) | Person-years | Observed deaths (n) | Person-years | Group 2 | Group 3 | ||||
| Total | 550 | 16,215 | 941 | 6,998 | 34 | 134 | 4.0 | ||
| Age (yr) | < 65 | Male | 43 | 4,862 | 165 | 2,375 | 9 | 69 | 7.9 |
| Female | 12 | 2,899 | 83 | 1,552 | 4 | 53 | 12.9 | ||
| ≥ 65 | Male | 311 | 4,510 | 383 | 1,697 | 69 | 226 | 3.3 | |
| Female | 184 | 3,944 | 310 | 1,374 | 47 | 226 | 4.8 | ||
Group 2, patients with diabetes or hypertension but without chronic kidney disease (CKD); group 3, patients with CKD.
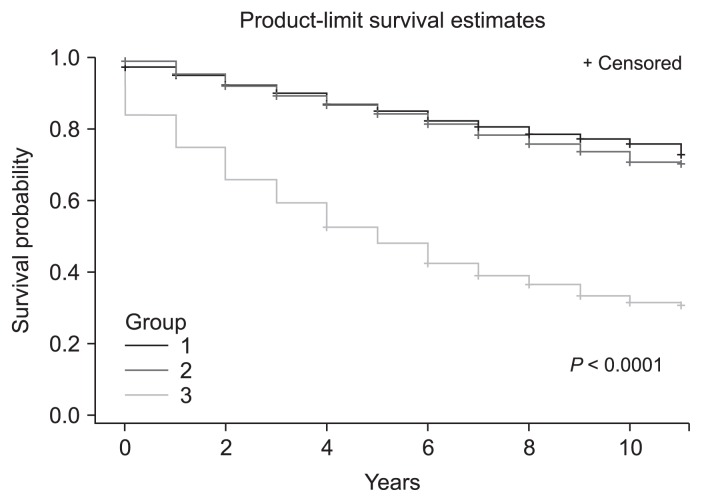
Figure 2. Kaplan–Meier survival curves for comparisons of survival rates by group (P < 0.0001 by log-rank test).
Group 1, control; group 2, patients with diabetes or hypertension but without chronic kidney disease (CKD); group 3, CKD patients.
A Cox proportional hazards analysis was performed for analysis of independent risk factors associated with all-cause mortality. Compared to the reference, group 3 had a significantly higher mortality than group 2 (group 3: hazard ratio [HR], 5.02; 95% confidence interval [CI], 4.49–5.62; group 2: HR, 1.14; 95% CI, 1.01–1.29, P < 0.001). Among subjects aged ≥ 65 years, the HR for mortality was 5.30 (95% CI, 4.70–5.96; P < 0.001) compared with subjects < 65 years, and the HR was 1.21 (95% CI, 1.10–1.32; P < 0.001) among male subjects compared with female subjects (Table 4).
Table 4.
Cox proportional hazard analysis for all-cause mortality
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Overall all-cause mortality | ||||||
| Group | ||||||
| 1 | Reference | Reference | ||||
| 2 | 1.13 | 1.00–1.28 | 0.050 | 1.14 | 1.01–1.29 | 0.040 |
| 3 | 4.37 | 3.91–4.89 | < 0.001 | 5.02 | 4.49–5.62 | < 0.001 |
| Age group (yr) | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 4.60 | 4.09–5.18 | < 0.001 | 5.30 | 4.70–5.96 | < 0.001 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.09 | 1.00–1.20 | 0.059 | 1.21 | 1.10–1.32 | < 0.001 |
| All-cause mortality between groups 1 and 3 | ||||||
| Group | ||||||
| 1 | Reference | Reference | ||||
| 3 | 4.30 | 3.85–4.81 | < 0.001 | 4.88 | 4.36–5.47 | < 0.001 |
| Age group | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 3.88 | 3.40–4.42 | < 0.001 | 4.51 | 3.95–5.15 | < 0.001 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.01 | 0.91–1.13 | 0.835 | 1.10 | 0.99–1.23 | 0.077 |
| All-cause mortality between groups 2 and 3 | ||||||
| Group | ||||||
| 2 | Reference | Reference | ||||
| 3 | 3.84 | 3.45–4.26 | < 0.001 | 4.36 | 3.92–4.85 | < 0.001 |
| Age group (yr) | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 3.87 | 3.41–4.40 | < 0.001 | 4.50 | 3.96–5.12 | < 0.001 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.11 | 1.00–1.23 | 0.056 | 1.21 | 1.09–1.35 | < 0.001 |
| Cardiovascular disease (yes) | 3.50 | 3.13–3.92 | < 0.001 | 2.00 | 1.78–2.25 | < 0.001 |
| Malignancy (yes) | 2.67 | 2.34–3.04 | < 0.001 | 1.73 | 1.51–1.98 | < 0.001 |
CI, confidence interval; HR, hazard ratio.
When comparing the two groups, we found that the HR of group 3 for mortality was 4.88 (95% CI, 4.36–5.47; P < 0.001) compared with group 1 (Table 4), and the HR of group 3 for mortality was 4.36 (95% CI, 3.92–4.85; P < 0.001) compared with group 2. The HRs for cardiovascular disease and malignancy were 2.00 (95% CI, 1.78–2.25; P < 0.001) and 1.73 (95% CI, 1.51–1.98; P < 0.001), respectively (Table 4).
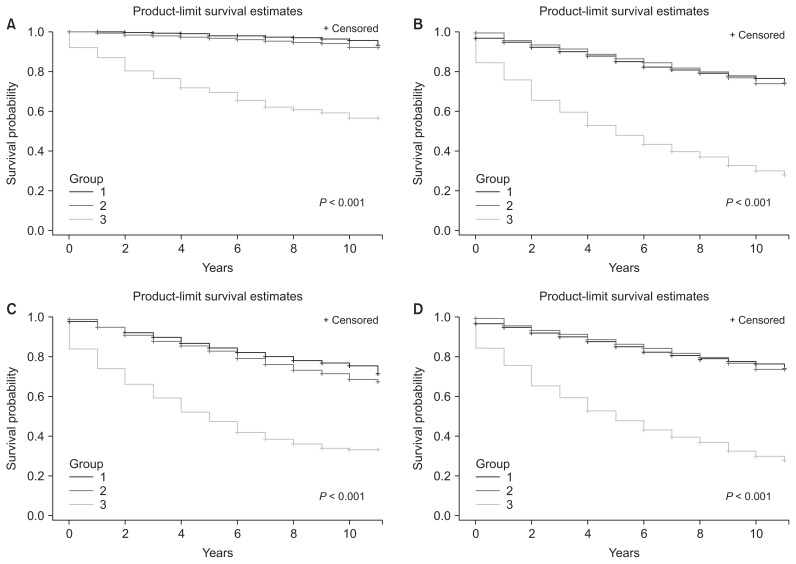
Kaplan–Meier survival curves with log-rank tests revealed that the survival rate in patients in group 3 was significantly lower than that in the other two groups, and the gap between the two curves widened over time (Fig. 2; P < 0.001). Fig. 3 shows the comparison of mortality rate by age or sex. Among patients in group 3, the cumulative survival rate was significantly lower in both patients older than 65 and patients younger than 65 (Fig. 3A, B). The survival rate was also significantly lower in group 3 compared to that in the other two groups irrespective of sex (Fig. 3C, D).
Figure 3. Kaplan–Meier survival curves for comparisons of survival rates by group (P < 0.001 by log-rank test).
(A) Survival curves for those under 65 by group, (B) survival curves for those 65 and older by group, (C) survival curves for men, (D) survival curves for women.
Group 1, control; group 2, patients with diabetes or hypertension but without chronic kidney disease (CKD); group 3, CKD patients.
Discussion
CKD is a major risk factor for all-cause mortality. Several epidemiologic studies have demonstrated that even mild elevation in serum creatinine level is associated with an increased rate of death from any cause [8–11]. A large observational study showed that the risk of death increased considerably as GFR decreased below 60 mL/min per 1.73 m2 [12]. One systematic review of the association between pre-dialytic CKD and the risk for all-cause mortality showed that the unadjusted relative risk of mortality in participants with versus without reduced kidney function ranged from 0.94 to 5.0 [3]. In another population-based study, the mortality rate was 120 deaths/1,000 person-years compared to controls matched from the general population, and the HR for mortality was 3.6 (95% CI, 3.2–4.0) for CKD [13]. These results demonstrate that CKD is a significant risk factor for death.
Some studies have revealed that racial and ethnic differences exist in mortality rates among individuals with CKD. Asians appear to have faster CKD progression and lower mortality rates compared to Caucasian populations [14–16]. Thus, it is important to build and manage data on the mortality of CKD for each country. In a recent observational cohort study, the overall age- and sex-adjusted standard mortality rate among Korean patients with ESRD was 10.3 (95% CI, 10.0–10.6) in 2009 and 10.9 (95% CI, 10.7–11.2) in 2010, respectively [17]. However, there is a lack of representative data on mortality in patients with CKD not undergoing dialysis.
In this population-based cohort study, we examined mortality among Korean patients with incident CKD in 2003 and 2007 using the NHIS-NSC database. The presence of CKD was associated with a significantly elevated risk for all-cause mortality in Korean patients; the mortality rate was 134 per 1,000 person-years, and the HR for mortality was 5.02 times higher than that of healthy controls and group 2 was 1.14 times higher than healthy controls.
In addition, we compared ‘patients with CKD’ with ‘patients with diabetes or hypertension, but without CKD’ to analyze only the effect of CKD on mortality, excluding the effects of diabetes or hypertension. As a result, the rate ratio of mortality was 4.0 and the HR for mortality was 4.36 times higher than that of group 2 (Table 4). Taken together, we conclude that CKD might be a significant risk factor for mortality irrespective of comorbid diseases such as diabetes and hypertension.
The mortality rate of patients with incident CKD calculated in this study was similar to or slightly higher than that of other studies mentioned above [3,13]. The reason may involve selection bias. We used a claims database and identified CKD with ICD-10 codes. Patients with advanced CKD, who had more comorbidities and used medical services more frequently, were more likely to have ICD codes indicating CKD. Moreover, we selected controls who were age- and sex-matched healthy individuals with a CCI of 0. This may explain why the difference between the mortality of CKD patients compared with controls in our study is higher than that in other studies.
In the present study, the mortality rates were higher in older CKD patients (≥ 65) than in younger CKD patients (< 65). However, the rate ratio for mortality appeared to be higher in the younger CKD patients compared with that in the older CKD patients. As seen in Table 2, the mortality rate in older CKD patients was much higher than that in the younger healthy population (226 vs. 69 in males; 226 vs. 53 in females), whereas the rate ratio for mortality in the older CKD patients was just lower than that in younger patients (4.0 vs. 13.5 in males and 4.6 vs. 20.9 in females). Moreover, when we investigated the crude death rate between groups 2 and 3 (Table 3), the rate ratios for mortality in younger patients were higher than those in older patients. We found that there was a relatively small number of death events in the younger healthy population and in younger patients with diabetes or hypertension, but without CKD (group 2) compared with those in the older healthy population and older patients in group 2. Thus, the higher mortality rate ratio might originate from the relatively lower mortality events in the younger healthy patients or group 2. The mean CCI score in the younger CKD patients was significantly lower than that in the older CKD patients (4.92 ± 2.53 vs. 6.05 ± 2.45, P < 0.001; Table 5).
Table 5.
Comparison of Charlson comorbidity index (CCI) score by age in the chronic kidney disease group
| CCI score
|
|||||
|---|---|---|---|---|---|
| n | Mean ± SD | Min | Max | P value | |
| Age group (yr) | <0.001 | ||||
| < 65 | 626 | 4.92 ± 2.53 | 2 | 15 | |
| ≥ 65 | 847 | 6.05 ± 2.45 | 2 | 17 | |
SD, standard deviation.
Our data had some limitations, and caution is necessary in interpreting these results. This study has similar inherent limitations as in other registry-based observational studies. First, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines define CKD as evidence of chronic kidney damage that lasts more than three months, even if the GFR is 60 mL/min per 1.73 m2 or higher. Albuminuria is the earliest marker of glomerular damage and is independently associated with increased mortality, even in the presence of normal GFR [9]. Therefore, subjects with albuminuria or other early markers of kidney damage and with normal or mildly decreased GFR (CKD stage 1 to 2) should be included in order to include its attributable mortality. However, since the patients with CKD enrolled in this study were assessed by reviewing diagnosis codes in claims, it is likely that patients with mild CKD were not included. Therefore, the number of patients with CKD may have been underestimated. However, we tried to define CKD based on previous epidemiologic studies [18–21], and to our knowledge there is no study of the impact of CKD on mortality compared with healthy controls and patients with diabetes or hypertension but without CKD. Second, considering the tendency of physicians not to enter CKD codes for patients with mild severity, it is likely that patients with more serious disease predominated in this study. This information can also be inferred from the fact that the proportions of patients with hypertension, diabetes, cardiovascular disease, and malignancy in this analysis were 90.6%, 56%, 26.2%, and 17.5%, respectively, which seems to be higher than expected in the general CKD population. Therefore, the mortality risk may have been overestimated. However, in a recent study, Kang et al [8] showed that 96.1% of patients with CKD registered in their cohort had hypertension, 5.3% had coronary artery disease, 3.5% had peripheral vascular disease, 6.0% had cerebrovascular disease, 1.5% had congestive heart failure, and 2.5% had arrhythmias. This data is similar to our findings. Third, the proportions of patients at each CKD stage in each group were not determined. Therefore, the impact of CKD stage could not be determined in a comparative analysis of mortality differences between groups.
Despite these limitations, the major strength of our present study is that it is the first study of mortality among patients with incident CKD and the impact of CKD on mortality. We found that Korean patients with CKD had higher mortality than healthy controls or patients with diabetes or hypertension but without CKD using a large and representative national sample. CKD might be a significant risk factor for mortality irrespective of comorbid diseases such as diabetes and hypertension. Future investigations are needed using large-scale cohort studies or complete enumeration involving more clearly separated groups of patients at all CKD stages and measuring renal function including albuminuria.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2010-0027945).
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Kyeong Min Kim and Hyung Jung Oh participated in the conception, study design, investigation, analysis, interpretation of data and wrote the manuscript. Hyung Yun Choi participated in the data curation and performed the statistical analysis. Hajeong Lee participated in the conception, validation and provided intellectual content of critical importance to the work. Dong-Ryeol Ryu participated in the onception, study design, interpretation of data, coordination, funding acquisition, project administration, supervision and writing editing. All authors read and approved the final manuscript.
References
- 1.Xie Y, Bowe B, Mokdad AH, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Lim CS, Han DC, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24(Suppl):S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 4.Dalrymple LS, Katz R, Kestenbaum B, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 7.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kang E, Han M, Kim H, et al. Baseline general characteristics of the Korean chronic kidney disease: report from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) J Korean Med Sci. 2017;32:221–230. doi: 10.3346/jkms.2017.32.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH. Predicting renal replacement therapy and mortality in CKD. Am J Kidney Dis. 2007;50:559–565. doi: 10.1053/j.ajkd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Neovius M, Jacobson SH, Eriksson JK, Elinder CG, Hylander B. Mortality in chronic kidney disease and renal replacement therapy: a population-based cohort study. BMJ Open. 2014;4:e004251. doi: 10.1136/bmjopen-2013-004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19:1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:101–107. doi: 10.2215/CJN.06450611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour SJ, Er L, Djurdjev O, Karim M, Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010;25:3663–3672. doi: 10.1093/ndt/gfq189. [DOI] [PubMed] [Google Scholar]
- 17.Choi H, Kim M, Kim H, et al. Excess mortality among patients on dialysis: comparison with the general population in Korea. Kidney Res Clin Pract. 2014;33:89–94. doi: 10.1016/j.krcp.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordunez P, Nieto FJ, Martinez R, et al. Chronic kidney disease mortality trends in selected Central America countries, 1997–2013: clues to an epidemic of chronic interstitial nephritis of agricultural communities. J Epidemiol Community Health. 2018;72:280–286. doi: 10.1136/jech-2017-210023. [DOI] [PubMed] [Google Scholar]
- 19.Kerr M, Matthews B, Medcalf JF, O’Donoghue D. End-of-life care for people with chronic kidney disease: cause of death, place of death and hospital costs. Nephrol Dial Transplant. 2017;32:1504–1509. doi: 10.1093/ndt/gfw098. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Jo MW, Go DS, Ryu DR, Park J. Economic burden of chronic kidney disease in Korea using national sample cohort. J Nephrol. 2017;30:787–793. doi: 10.1007/s40620-017-0380-3. [DOI] [PubMed] [Google Scholar]
- 21.Oh HJ, Lee HA, Moon CM, Ryu DR. Incidence risk of various types of digestive cancers in patients with pre-dialytic chronic kidney disease: a nationwide population-based cohort study. PLoS One. 2018;13:e0207756. doi: 10.1371/journal.pone.0207756. [DOI] [PMC free article] [PubMed] [Google Scholar]