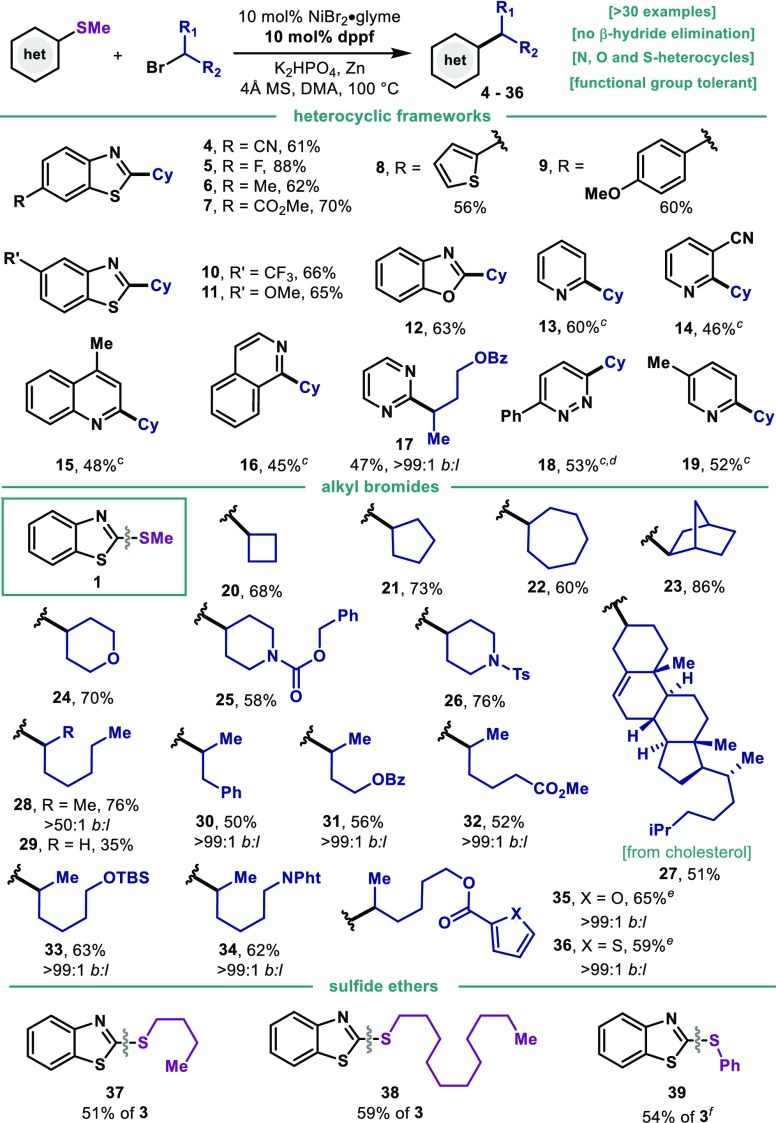
Table 2. Scope of the Reductive Liebeskind–Srogl Alkylationa,b.
Thioether (1 equiv, 0.2 mmol), alkyl bromide (2.0 equiv), NiBr2·diglyme (10 mol %), dppf (10 mol %), K2HPO4 (2.0 equiv), Zn (2.5 equiv), 4 Å MS, DMA (0.6 mL) at 100 °C, 6 h.
Isolated yields.
Alkyl bromide (3.0 equiv).
50 °C, 24 h.
12 h.
Traces of alkylation at Ph–S cleavage observed by GC-MS.