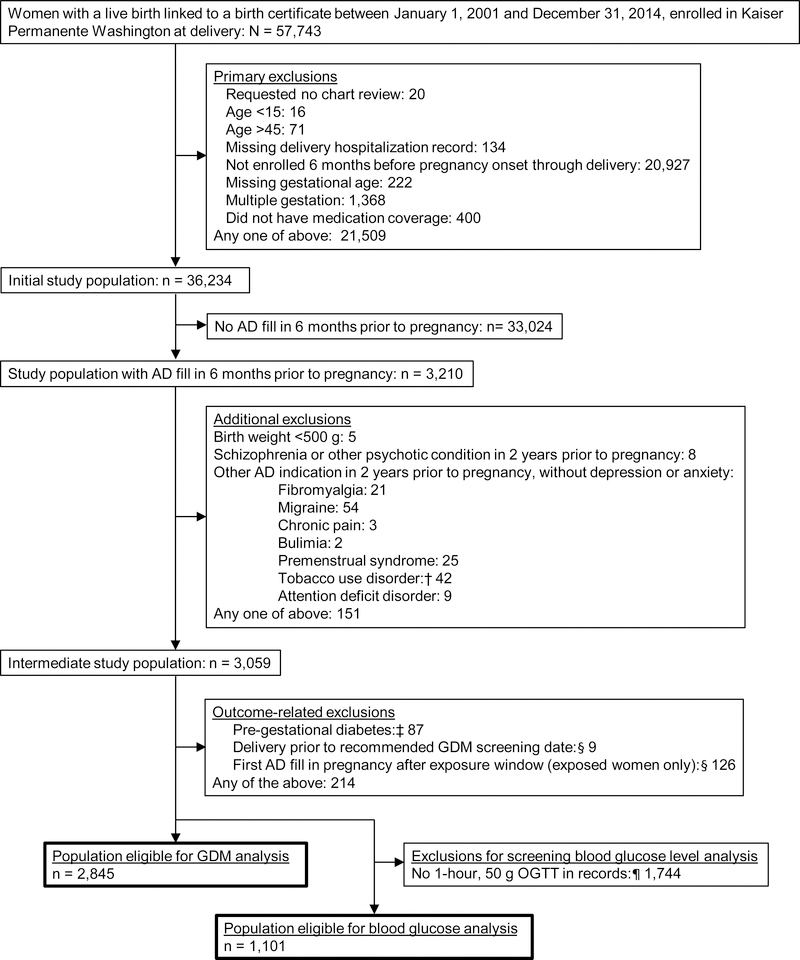
Figure 1. Identification of the study population.
AD: Antidepressant; GDM: Gestational diabetes; OGTT: Oral glucose tolerance test; IGP: Integrated Group Practice
†We only excluded deliveries to women with a tobacco use disorder code in the 2 years prior to pregnancy if they also had a prescription fill for bupropion, the only antidepressant indicated to treat tobacco use disorder.
‡Women with type 1 or type 2 diabetes are, by definition, not at risk for GDM; we excluded women with those diagnostic codes (Supplementary Table 2) recorded between six months prior to pregnancy and 24 weeks gestation.
§The exposure window spans the start of pregnancy through 4 weeks before GDM screening. For the 8% of women missing GDM screening date from a procedure code, we assumed they were screened at 28 weeks, based on screening guidelines. If these women delivered at or before 28 weeks gestation, we excluded them, because they may not have received GDM screening.
¶We expected 1-hour, 50 g OGTTs (part of the 1-step testing strategy) to only be present in our laboratory data for women in the IGP from 2001–2011, after which the IGP switched to the 2-step testing strategy, which used 2-hour, 75-g OGTTs (Methods Appendix 1).