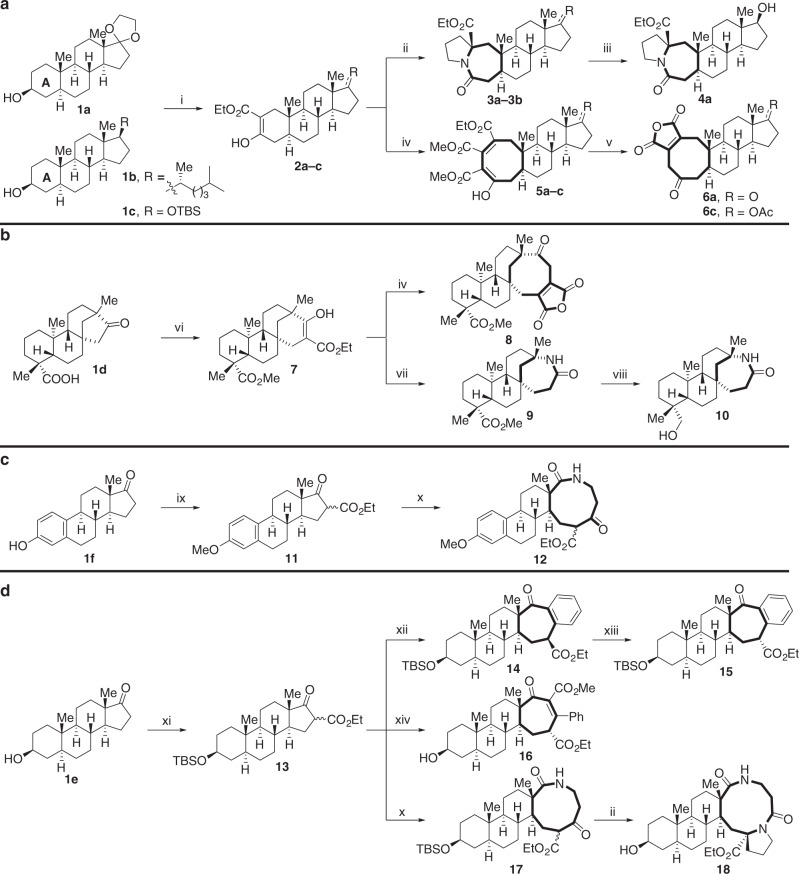
Fig. 2.
Ring expansion of polycyclic natural products based on native C–O bonds. a A-ring expansion of dehydroepiandrosterone and cholesterol. b D-ring expansion of isosteviol. c D-ring expansion of estrone. d D-ring expansion of dehydroepiandrosterone. (i) (a) TPAP, NMO, 4 Å MS, CH2Cl2, (b) LDA, THF, then CNCO2Et, −78 °C; (ii) (a) NaH, HMPA, THF, rt, then 1-Chloro-3-iodopropane, rt, (b) NaN3, DMF, 80 °C, (c) CF3COOH, rt; (iii) NaBH4, MeOH, −78 °C; (iv) NaH, toluene, then DMAD, rt; (v) HCl, AcOH, 120 °C; (vi) (a) Me2SO4, LiOH, THF, 65 °C, (b) Ethyl diazoacetate, BF3•Et2O, Et2O/CH2Cl2, rt; (vii) (a) LiCl, DMSO, H2O, 120 °C, (b) NH2OH•HCl, KOAc, 70 °C, (c) TsCl, DMAP, Py, 60 °C; (viii) Dibal-H, CH2Cl2, −78 °C; (ix) (a) NaOH, Me2SO4, acetone, 60 °C, (b) LDA, THF, −78 °C, then CNCO2Et; (x) (a) MgCl2, Py, NHCbz(CH)2COCl, CH2Cl2, (b) Pd/C, H2, EtOAc, rt; (xi) (a) TBSCl, imidazole, CH2Cl2, rt, (b) LDA, THF, then CNCO2Et, −78 °C; (xii) 2-(Trimethylsilyl)phenyl trifluoromethanesulfonate, CsF, MeCN, 80 °C, 18:1 d.r.; (xiii) TBAF, THF, rt; (xiv) (a) NaH, THF, methyl phenylpropiolate, 65 °C, (b) TsOH, THF, rt. Full details are in the Supplementary Figures 5–7