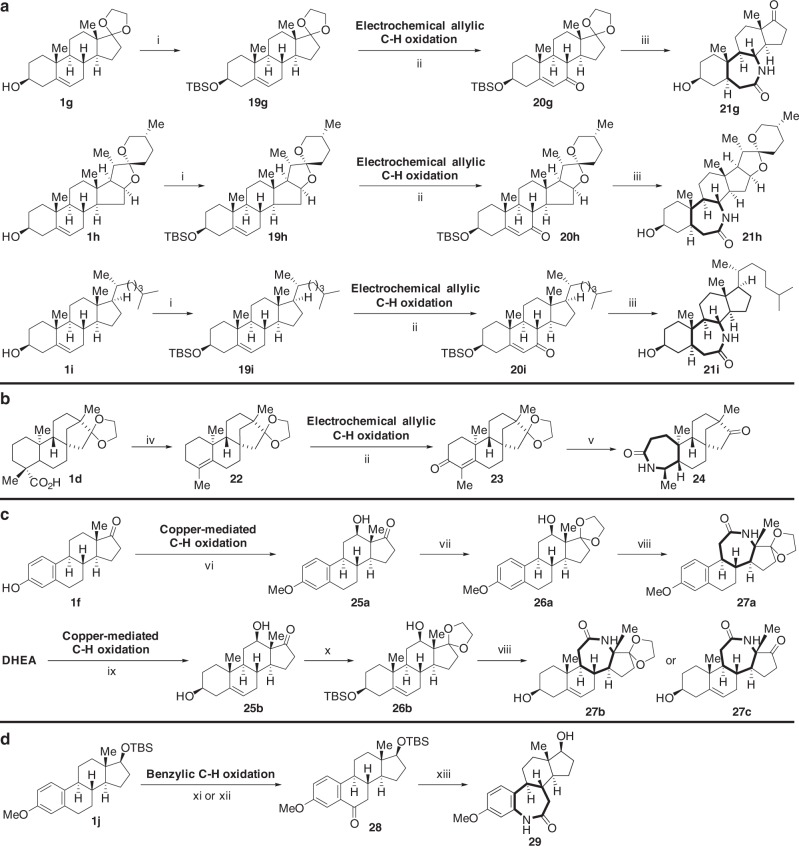
Fig. 3.
Diversification of polycyclic natural products by sequential C–H oxidation and ring expansion. a Electrochemical C–H oxidation/B-ring expansion of DHEA, cholesterol and Diosgenin. b Electrochemical C–H oxidation/A-ring expansion of isosteviol. c Copper-mediated C–H oxidation/C-ring expansion of DHEA and estrone. d Benzylic C–H oxidation/C-ring expansion of estrone. (i) TBSCl, imidazole, CH2Cl2, rt; (ii) LiClO4, Py, t-BuOOH, Cl4NHPI, acetone, rt; (iii) (a) Pd/C, H2, EtOAc, rt, (b) NH2O•HCl, KOAc, 70 °C, (c) TsCl, DMAP, Py, 60 °C; (d) TsOH, THF/H2O or Pd/C, MeOH, rt; (iv) (a) Pb(OAc)4, Cu(OAc)2, Py, toluene, 90 °C, (b) I2, toluene, 120 °C (c) glycol, toluene, TsOH, 120 °C; (v) (a) PtO2, H2, rt, (b) NaOMe, MeOH, rt, (c) NH2OH•HCl, KOAc, 70 °C, (d) TsCl, DMAP, Py, 60 °C; (vi) (a) Me2SO4, K2CO3, acetone, (b) TsOH, toluene, (4-methylpyridin-2-yl)methanamine, 120 °C, (c) [Cu(MeCN)4PF6], O2, (+)-sodium-(L)-ascorbate, acetone/MeOH, 50 °C, 6 h, then, Na4EDTA, rt; (vii) glycol, toluene, TsOH, 120 °C; (viii) (a) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C (b) NH2OH•HCl, KOAc, 70 °C, (c) TsCl, DMAP, Py, 60 °C, (d) TsOH, THF/H2O, rt, for 27c, 60 °C, 5 days; (ix) (a) TsOH, toluene, (4-methylpyridin-2-yl)methanamine, 120 °C, (b) [Cu(MeCN)4PF6], O2, (+)-sodium-(L)-ascorbate, acetone/MeOH, 50 °C, 6 h, then, Na4EDTA, rt; (x) (a) glycol, toluene, TsOH, 120 °C, (b) TBSCl, imidazole, CH2Cl2, rt; (xi) Cr(CO)6, tBuOOH, MeCN, 70 °C; (xii) (a) KtOBu, LDA, (MeO)3B, THF, (b) H2O2, NaOH, (c) (COCl)2, DMSO, Et3N, CH2Cl2; (xiii) (a) NH2OH•HCl, KOAc, EtOH, (b) TsCl, DMAP, Py, (c) CF3COOH. Full details are in the Supplementary Figures 8–11