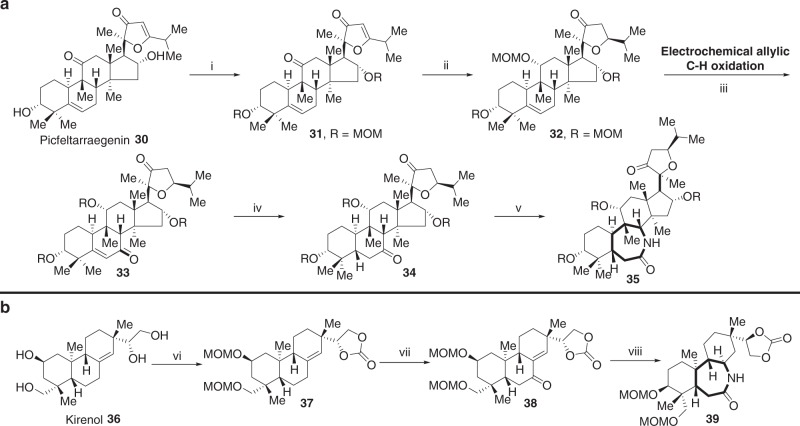
Fig. 4.
Application of the two-phase C–H oxidation/ring expansion strategy to picfeltarraegenin and kirenol. a Electrochemical allylic C–H oxidation/ring expansion of picfeltarraegenin. b Allylic C–H oxidation/ring expansion of kirenol. (i) MOMCl, DIPEA, DMAP, CH2Cl2, rt; (ii) (a) NaBH4, EtOH, rt, (b) MOMCl, DIPEA, DMAP, CH2Cl2, 50 °C; (iii) LiClO4, Py, t-BuOOH, Cl4NHPI, acetone, rt. (iv) Pd/C, H2, MeOH, rt; (v) NH2OH•HCl, KOAc, (c) TsCl, DMAP, Py, 60 °C; (vi) (a) CDI, toluene, 90 °C, (b) MOMCl, DIPEA, DMAP, CH2Cl2, rt; (vii) (a) SeO2, dioxane, (b) (COCl)2, DMSO, Et3N, CH2Cl2; (viii) (a) Pd/C, H2, EtOAc, (b) NH2OH•HCl, KOAc, 70 °C, (c) TsCl, DMAP, Py, 60 °C. Full details are in Supplementary Figure 12