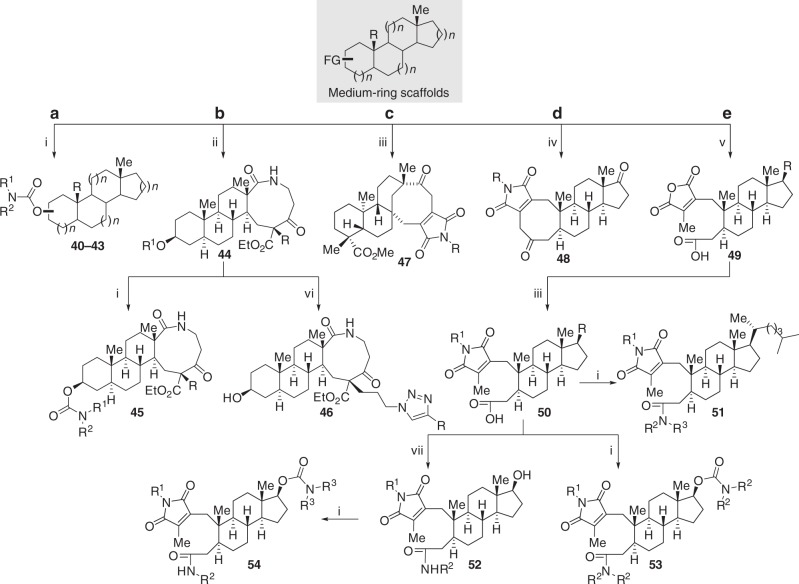
Fig. 5.
Further diversication of medium-sized ring scaffolds. a Formation of carbamates. b Formation of azide. c. Formation of imide 47. d Formation of imide 48. e Ring-cleavage of medium ring scaffold. A total of 150 polycyclic final products, most of which have >90% purity (LC-MS, ultraviole detector at 254 or 210 nm) and >10 mg quantities, were prepared. (i) (a) CDI, Et3N, CH2Cl2, rt, (b) R1R2NH, DMAP, toluene, 90 °C; (ii) NaH, HMPA, THF, then RI, rt; (iii) R1R2NH, toluene/CH2Cl2, rt, then 90 °C; (iv) (a) 5 M NaOH, THF, 60 °C, then NaBH4, rt, then HCl, (b) RNH2, toluene, rt, then 90 °C, (c) Dess-Martin oxidant, NaHCO3, CH2Cl2; (v) (a) NaOH, EtOH/H2O, 100 °C, (b) DMSO, HCl, 120 °C; (vi) (a) NaN3, DMF, 80 °C, (b) Sodium ascorbate, CuSO5•H2O, HCCR, THF/H2O, rt, (c) TsOH, THF/H2O, rt; (vii) DPPA, Et3N, R2NH2, DMF, rt. Full details are in the Supplementary Figures 13–16