Abstract
The integrity of the blood–brain barrier (BBB) plays a vital role in affecting the prognosis of subarachnoid hemorrhage (SAH). This study aimed to investigate activation of the Tropomyosin-related kinase receptor B (TrkB) and its downstream signaling pathway on preserving BBB breakdown after experimental SAH. An endovascular perforation SAH model was applied. N-[2-(5-hydroxy-1H-indol-3-yl) ethyl]-2- oxopiperidine-3-carboxamide (HIOC), the derivative of N-acetyl serotonin (NAS), was intracerebroventricularly administered 3 h after SAH induction. The neurologic scores and brain water content were evaluated in an outcome study. Western blot and immunofluorescence staining were used to investigate the mechanism. The results indicated that HIOC activated the TrkB/Akt pathway, increased the tight junction expression, improved neurologic deficits, and ameliorated brain edema after SAH. Thus, we conclude that initiating the TrkB/Akt signaling cascade preserves BBB breakdown after experimental SAH in rats.
Keywords: subarachnoid hemorrhage, tropomyosin-related kinase receptor b, early brain injury, blood–brain barrier, tight junction
Introduction
Subarachnoid hemorrhage (SAH) is a devastating cerebral vascular disease in neurosurgery departments, with an incidence of 6–8 per 100,000 per year1. The average mortality of SAH is approximately 51%, and 33% of survivors require lifetime medical care2,3. Although microsurgical and endovascular techniques have rapidly changed in the past few decades, the mortality and disability rates of SAH have not fundamentally changed.
Disruption of the blood–brain barrier (BBB) is one of the pathophysiological changes in the early stage after a SAH ictus. A substantial number of clinical and experimental studies have indicated that BBB degradation plays a pivotal role in leading to delayed cerebral ischemia and delayed ischemic neurological deficits, which are highly associated with the poor prognosis after SAH4,5.
Tropomyosin-related kinase receptor B (TrkB), a member of the receptor tyrosine kinase family6, has been proven to ameliorate the early brain injury after experimental SAH7. In a previous study, N-[2-(5-hydroxy-1H-indol-3-yl) ethyl]-2- oxopiperidine-3-carboxamide (HIOC), the derivative of N-acetyl serotonin (NAS), was confirmed to be a potent agonist of TrkB8. Moreover, we have demonstrated that HIOC can effectively activate TrkB following induction of the SAH model and attenuate neuronal apoptosis9. However, the effects of HIOC on BBB preservation have not been discussed.
The present study aimed to investigate the potential role of HIOC on BBB protection and its underlying mechanisms in a SAH rodent model.
Materials and Methods
Animals and Experimental Design
All animal procedures were approved by the Committee of Animal Use for Research at Shanghai Jiao Tong University School of Medicine (China). Surgeries were performed on male Sprague-Dawley rats that weighed 280–320 g.
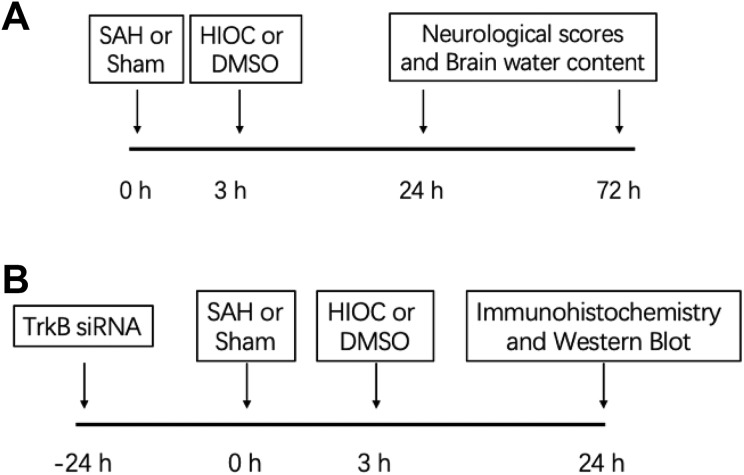
In the outcome experiment (Fig. 1A), 58 rats were randomly divided into sham-operated (n=12), SAH (n=15), SAH + Vehicle (DMSO) (n=15), and SAH + HIOC (n=16) groups. Three hours after SAH was induced, vehicle or HIOC was intracerebroventricularly injected. The neurological scores and brain water content were assessed at the 24 and 72 h time points. Treatment with HIOC 3 h after SAH was determined by our previous study9.
Fig. 1.
Scheme of the study design. (A) Design of the outcome study. (B) Design of the mechanism study.
In the mechanism experiment (Fig. 1B), 29 rats were assigned to four groups: sham-operated (n=7), SAH + Vehicle (DMSO) (n=8), SAH + HIOC (n=7), and TrkB siRNA + SAH + HIOC (n=7). TrkB siRNA was intracerebroventricularly injected 24 h prior to SAH. Immunofluorescence staining and the protein levels of phosphorylated TrkB (p-TrkB), p-Akt, p-GSK3β, and ZO-1 were detected 24 h after SAH.
SAH Model Induction and Intracerebroventricular (icv) Injection
SAH was induced by endovascular perforation according to our previous study10. All animals were anesthetized with 5% isoflurane in 70/30% medical air/oxygen and maintained with 3% isoflurane in the same ratio of mixed air and oxygen after transoral intubation with a tracheal cannula, which was subsequently connected to a small respirator to assist respiration.
The external carotid artery was exposed and ligated into a 3 mm stump, through which a 4-0 sharpened nylon suture was threaded into the internal carotid artery (ICA) and advanced into the Circle of Willis at the bifurcation of the anterior cerebral artery and middle cerebral artery. SAH was produced by ICA reperfusion after the suture was then pulled back. The sham-operated rats received the same surgical process without puncture of the intracranial branch point.
HIOC (Bio-Techne, Shanghai, China) (30 μg in 5 μl of DMSO), vehicle (DMSO) (5 μl) and TrkB siRNA (500 pmol/5 μl) (Santa Cruz Biotechnology, Dallas, TX, USA) were intracerebroventricularly injected as previously described11. The rats were placed on the stereotaxic apparatus. HIOC, vehicle, or TrkB siRNA was slowly stereotaxically injected into the left lateral ventricle at the coordinates of 1.5 mm posterior and 1.0 mm lateral to the bregma and 4.0 mm ventral to the cortical surface.
TrkB Silencing
The following sequences were used to design TrkB siRNA:
(a) sense, 5′-GGAUUCCGGCUUAAAGUUU-3′,
antisense, 5′-AAACUUUAAGCCGGAAUCC-3′;
(b) sense, 5′-GGAUUUGUAUUGCCUCAAU-3′,
antisense, 5′-AUUGAGGCAAUACAAAUCC-3′;
(c) sense, 5′-CCAUCACAUU UCUCGAAUC-3′,
antisense, 5′-GAUUCGAGAAAUGUGAUGG-3′.
Neurologic Outcome Assessment
The neurologic scores were assessed at 24 and 72 h after SAH onset by means of a modified Garcia scale (3–18 points) and beam balance test (0–4 points)12. The modified Garcia scale consists of six tests, which include spontaneous activity, symmetry movement of four limbs, forelimbs outstretching, climbing, body proprioception, and response to vibrissae.
SAH Severity Score
After the rats were euthanized, the brain was dissected, and the basal cistern was divided into six segments. Each area was scored from 0 to 3 depending on the amount of subarachnoid blood clot13. SAH grading was obtained by summing all six segments’ scores (0–18 points).
Brain Water Content
The rat’s brain was rapidly divided into four parts (left hemisphere, right hemisphere, cerebellum, and brain stem) and weighed to obtain the wet weight (WW). After the sample was dehydrated (105°C for 72 h), the brain sample was weighed again to acquire the dry weight (DW). The brain water content was calculated as [(WW–DW)/WW] ∗ 100%14.
Western Blot Analysis
The left hemisphere (perforation side) was isolated and collected for Western blot analysis as previously described9. The following primary antibodies were used for immunoblotting: anti-phosphorylated TrkB (1:1000, Abcam, Cambridge, MA, USA), anti-phosphorylated Akt, anti-phosphorylated GSK3β (1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-ZO-1 and anti-β-actin (1:500, Santa Cruz Biotechnology).
Double Immunofluorescence Staining
Fixed frozen sections were created as previously described15. The sections were incubated with primary antibodies (anti-ZO-1, Abcam, and anti-von Willebrand factor, Millipore, Temecula, CA, USA) at 4°C overnight, followed by secondary antibodies (fluorescein isothiocyanate and Texas Red, Jackson Immunoresearch, West Grove, PA, USA) for 2 h at room temperature. Immunofluorescence was observed under a fluorescence microscope (Olympus OX51, Tokyo, Japan).
Statistical Analysis
All data are presented as the means ± standard error of the means. One-way analysis of variance with Tukey’s post-hoc test was applied for multiple comparisons among groups using GraphPad Prism Software (CA, USA). p < 0.05 was considered statistically significant.
Results
Mortality and SAH Grading
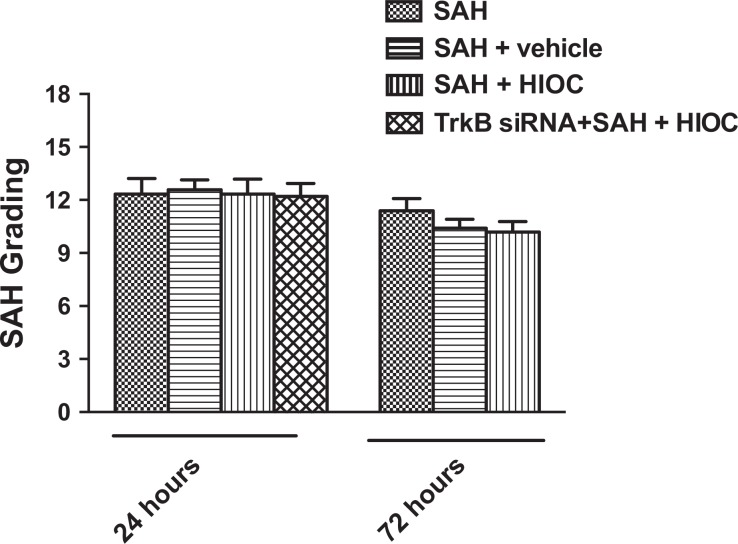
The mortality was similar among the SAH groups in this study (SAH 3/15, SAH+Vehicle 3/15, SAH+HIOC 4/16, and TrkB siRNA + SAH + HIOC 1/7). There was no significant difference in the SAH severity score among the SAH, SAH+Vehicle, SAH+HIOC, and TrkB siRNA+SAH+HIOC groups at the 24 and 72 h time points (Fig. 2).
Fig. 2.
SAH grading among SAH groups at 24 and 72 h time points. There is no significant difference between groups (p > 0.05).
HIOC Treatment Attenuated Neurologic Deficits and Brain Edema after SAH
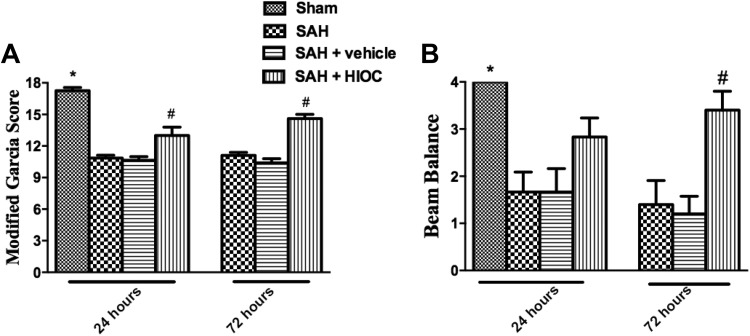
After SAH was induced, the modified Garcia and beam balance scores were significantly impaired at both the 24 and 72 h time points. The administration of HIOC ameliorated the neurologic deficits in the modified Garcia test at the 24 and 72 h time points (Fig. 3A). The beam balance score was elevated following HIOC treatment at 72 h after SAH onset, while there was a trend that the beam balance increased after the SAH rats were injected with HIOC at the 24 h time point (Fig. 3B).
Fig. 3.
Neurologic function assessment at 24 and 72 h after SAH and sham groups (n = 6 per group). (A) ∗p<0.05 vs. SAH groups, #p<0.05 vs. SAH and SAH+vehicle groups. (B) ∗p<0.05 vs. SAH and SAH+vehicle groups, #p < 0.05 vs. SAH and SAH+vehicle groups.
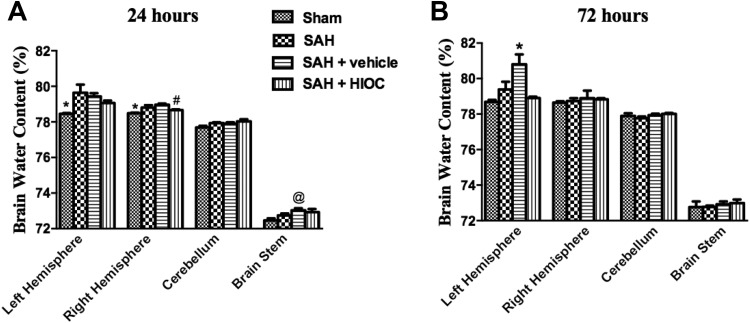
At the 24 h time point, brain edema was significantly elevated in the supratentorial hemisphere, and there was no difference in the brain water content between the sham and SAH+HIOC groups. HIOC treatment attenuated the brain water content in the right hemisphere (Fig. 4A). At 72 h after SAH was induced, the brain water content in the vehicle group was significantly higher than that in the sham and HIOC injection groups in the left hemisphere (Fig. 4B).
Fig. 4.
Brain water content of rat brain regions in SAH and sham groups at 24 (A) and 72 h (B) time points (n = 6 per group). (A) ∗p<0.05 vs. SAH and SAH+vehicle groups. #p < 0.05 vs. SAH+vehicle group. @p < 0.05 vs. sham group. (B) ∗p<0.05 vs. sham and SAH+HIOC groups.
HIOC Preserved BBB Integrity
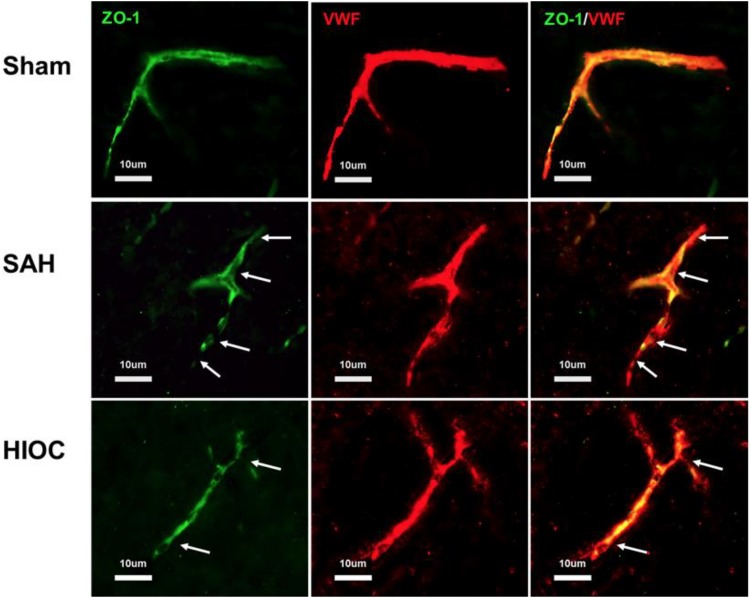
The integrity of the BBB was detected by means of double immunofluorescence staining. Intact endothelial cells (vWF) and ZO-1 were observed in the sham rats. The integrity was disrupted at 24 h after SAH induction, while the structures were preserved by HIOC treatment (Fig. 5).
Fig. 5.
Double immunofluorescence staining of vWF and ZO-1 at 24 h after SAH. Arrow indicates the breakdown of the continuous endothelial cell layer.
HIOC Activated TrkB/Akt/GSK-3β Signaling Cascade
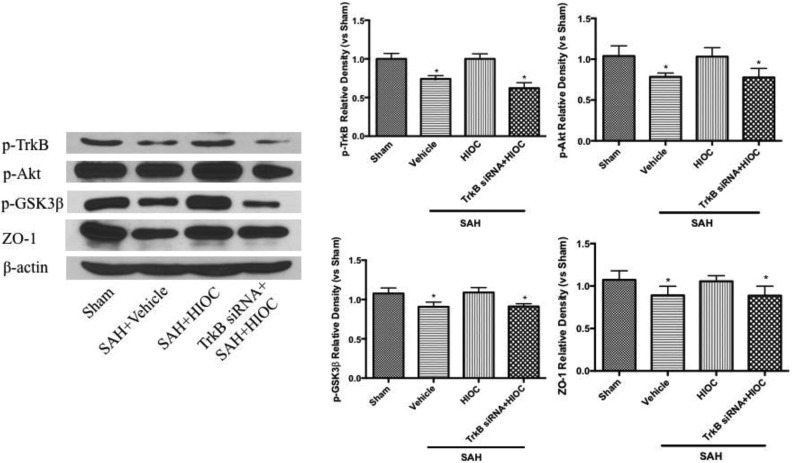
Western blot analyses showed that the phosphorylation of TrkB and its downstream effectors phosphorylated Akt and GSK-3β were significantly decreased at 24 h after SAH. The tight junction protein ZO-1 expression was also reduced in the vehicle group. HIOC administration significantly elevated the level of phosphorylated TrkB and its subsequent signaling pathway, and the level of ZO-1 expression was also higher in the treatment group. These results were reversed by TrkB siRNA pretreatment (Fig. 6).
Fig. 6.
Western blot analyses of the expression of TrkB and its downstream regulators (n=6 per group). ∗p < 0.05 vs. sham and SAH+HIOC groups.
Discussion
Our study indicated the role of TrkB-related cell signaling on preserving BBB disruption after hemorrhagic stroke. Moreover, HIOC was proved to be a neuroprotective agent by attenuating brain edema in experimental SAH rats.
The BBB breakdown causes poor prognosis through secondary injury. The tight junction disruption leads to exudate full of protein diffusing into the extracellular space of the brain and elevated the osmotic pressure, which further causes extracellular fluid accumulation. Moreover, the brain swelling increases the intracranial pressure and lowers the cerebral perfusion pressure and cerebral blood flow16. A previous study showed that diffuse brain swelling, which is an independent risk factor for death and poor outcome, appeared in approximately 8% of SAH patients in the early stage of the disease and would occur in 12% of SAH patients in the following course of the disease4. Moreover, inflammatory cells from peripheral blood can penetrate into the central nervous system through a corrupted BBB. Following infiltration, pro-inflammatory cytokines, cytotoxic proteases, and reactive oxygen species contribute to an excessive inflammatory response, which is closely related to apoptosis and epilepsy. Therefore, BBB preservation after SAH could be a potential therapeutic target.
In our previous study, we demonstrated that the activation of TrkB after SAH can decrease early brain injury by attenuating neuronal apoptosis9. However, the neuroprotective role of TrkB on preserving BBB integrity has not been explored. It has been proven that a high dosage of HIOC treatment 3 h after SAH occurred can significantly improve neurologic function9. Thus, we used the dosage of 30 μg and the icv injection time point of 3 h for the present study.
Shen et al. have shown that HIOC exerts its neuroprotective role by provoking TrkB and its downstream effector Akt8. In our study, we also demonstrated that the phosphorylation of TrkB and Akt were upregulated by HIOC administration in the SAH model. The activity of GSK-3β can be inhibited by increasing its phosphorylation through the PI3K/Akt pathway17. Thus, we assessed the level of phosphorylated GSK-3β and found it was increased after HIOC injection. However, this effect was prevented by TrkB siRNA. Active GSK-3β can negatively regulate cytosolic β-catenin by targeting its ubiquitination and degradation18. Phosphorylation of GSK-3β can trigger cytosolic β-catenin accumulation and promote β-catenin nuclear translocation19. Our results indicated that the tight junction protein ZO-1 increased after HIOC treatment, which may be associated with the activation of the classical Wnt/β-catenin signaling cascade. After β-catenin’s nuclear translocation and interaction with transcription factor, it could enhance the tight junction such as Occludin and ZO-1 transcription and translation20. Thus in future research we need to investigate the β-catenin level changes in both cytoplasm and nuclear, as well as the mRNA level alteration of tight junction, to clarify the underlying mechanisms.
Conclusion
HIOC acts as a potential neuroprotective agent following SAH by activating the TrkB/Akt/GSK-3β pathway and attenuating BBB disruption.
Footnotes
Ethical Approval: All animal procedures in this study were approved by the Committee of Animal Use for Research at Shanghai Jiao Tong University School of Medicine (China).
Statement of Human and Animal Rights: All animal procedures in this study were conducted in accordance with the Committee of Animal Use for Research at Shanghai Jiao Tong University School of Medicine (China) approved protocols.
Statement of Informed Consent: There are no human subjects in this article, and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Grants from the National Natural Science Foundation of China 81601013 to Junjia Tang and China Postdoctoral Science Foundation Grant 2018M632130 to Jianfei Lu.
ORCID iD: Junjia Tang  https://orcid.org/0000-0002-1020-7748
https://orcid.org/0000-0002-1020-7748
References
- 1. Bogason ET, Anderson B, Brandmeir NJ, Church EW, Cooke J, Davies GM, Hussain N, Patel AS, Payne R, Rohatgi P, Sieg E, et al. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2014;74(2):227–229. [DOI] [PubMed] [Google Scholar]
- 2. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–318. [DOI] [PubMed] [Google Scholar]
- 3. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354(4):387–396. [DOI] [PubMed] [Google Scholar]
- 4. Caner B, Hou J, Altay O, Fujii M, Zhang JH. Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem. 2012;123(suppl 2):12–21. [DOI] [PubMed] [Google Scholar]
- 5. Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97(1):14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulle F, Kenis G, Cazorla M, Hamon M, Steinbusch HW, Lanfumey L, van den Hove DL. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol. 2012;98(2):197–206. [DOI] [PubMed] [Google Scholar]
- 7. Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42(2):477–483. [DOI] [PubMed] [Google Scholar]
- 8. Shen J, Ghai K, Sompol P, Liu X, Cao X, Iuvone PM, Ye K. N-acetyl serotonin derivatives as potent neuroprotectants for retinas. Proc Natl Acad Sci U S A. 2012;109(9):3540–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang J, Hu Q, Chen Y, Liu F, Zheng Y, Tang J, Zhang J, Zhang JH. Neuroprotective role of an N-acetyl serotonin derivative via activation of tropomyosin-related kinase receptor B after subarachnoid hemorrhage in a rat model. Neurobiol Dis. 2015;78:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Ma Q, Krafft PR, Hu Q, Rolland W, 2nd, Sherchan P, Zhang J, Tang J, Zhang JH. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis. 2013;58:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol. 2010;68(5):650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634. [DOI] [PubMed] [Google Scholar]
- 13. Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167(2):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41(8):1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L, Wang K, Chen L, Huang H, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216(1):35–46. [DOI] [PubMed] [Google Scholar]
- 16. Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. [DOI] [PubMed] [Google Scholar]
- 18. Ramirez SH, Fan S, Dykstra H, Rom S, Mercer A, Reichenbach NL, Gofman L, Persidsky Y. Inhibition of glycogen synthase kinase 3β promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. Plos One. 2013;8(2):e55972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang WC, Tsai JJ, Kuo CY, Chen HM, Kao SH. Non-proteolytic house dust mite allergen, Der p2, upregulated expression of tight junction molecule claudin-2 associated with Akt/GSK-3β/β-catenin signaling pathway. J Cell Biochem. 2011;112(6):1544–1551. [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo C, Tang J, Feng H, Zhang JH. Norrin protected blood-brain barrier via frizzled-4/β-catenin pathway after subarachnoid hemorrhage in rats. Stroke. 2015;46(2):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]