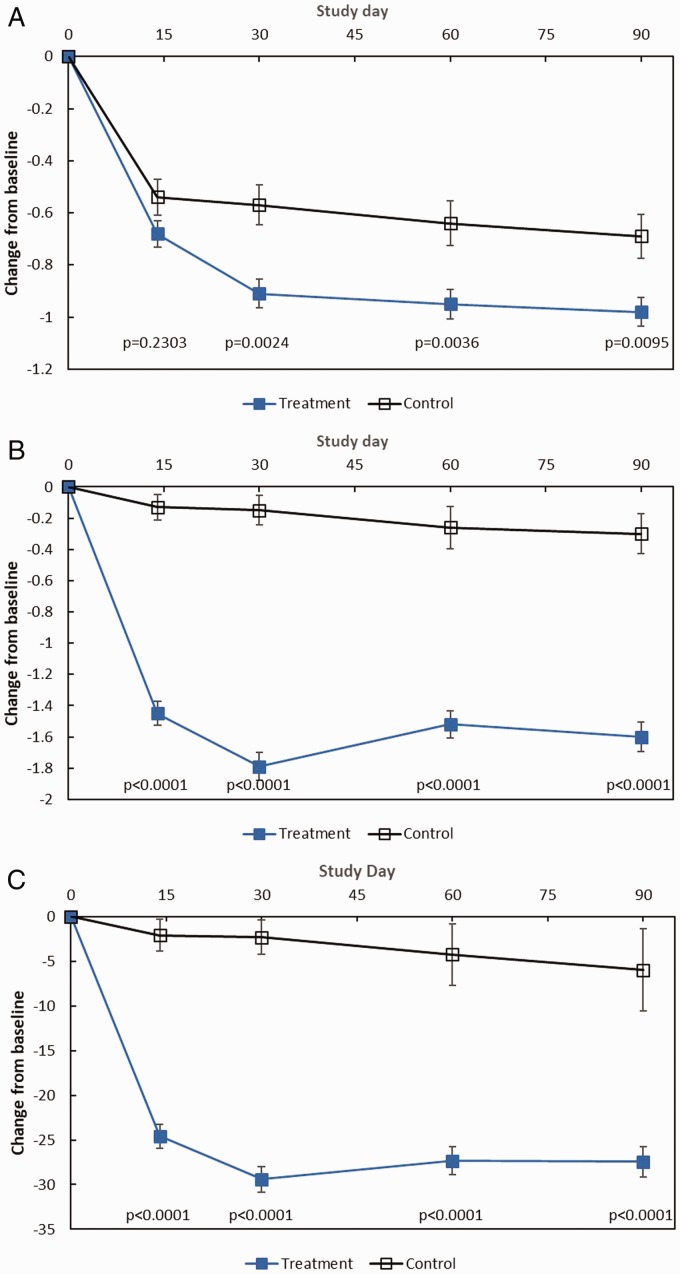
Figure 1.
Pooled analysis efficacy outcomes through day 90. The changes from baseline to each time point in subjective and objective outcomes among treatment patients who received MF sinus implants bilaterally and control patients who underwent a sham procedure are shown. All patients (treatment and control) were required to use MFNS once daily. A, Change in NO/C (scale 0–3, with higher score indicating greater severity), as assessed by patients using a reflective questionnaire. B, Change in BPG (scale 0–8, with higher grade indicating greater severity), as assessed by clinical investigators. C, Change in ESO (VAS 0–100, with higher score indicating greater severity), as assessed by clinical investigators. All values are means with 2-sided standard error bars calculated based on ITT population. Data from patients who received surgical or medical intervention were imputed using most recent values prior to initiating or receiving intervention and represent intervention-adjusted values. P values based on the ANCOVA model with baseline value as a covariate and study, site, and treatment group as fixed effects. ANCOVA, analysis of covariance; BPG, bilateral polyp grade; ESO, ethmoid sinus obstruction; ITT, intent-to-treat; MF, mometasone furoate; MFNS, MF nasal spray; NO/C, nasal obstruction/congestion; VAS, visual analog scale.