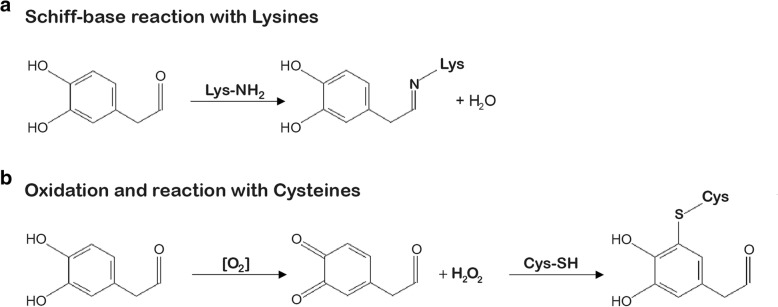
Fig. 2.
DOPAL reactivity and reported neurotoxic molecular mechanisms. DOPAL reactivity is due to both the aldehyde and the catechol moiety, respectively resulting in covalent modification of primary amines and thiols (i.e. lysine and cysteine residues of proteins) [36–38]. a DOPAL addiction to lysines is the result of a Schiff-base reaction between the aldehyde and the primary amine of the lysine’s lateral chain, with the release of a molecule of water. b In oxidative conditions, the catechol group has the tendency to auto-oxidation, with production of quinones and oxygen radical species [39]. Also, the oxidized cathecol is reactive towards the thiols of cysteines