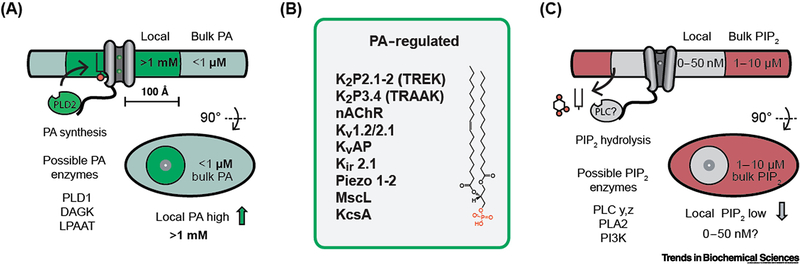
Figure 4. Lipid activation of ion channels through localized synthesis.
(A) Co-localization of TREK-1 with phosphatidic acid (PA) very convincingly established a lipid-protein based localization mechanism as a means of its activation [40]. Indirectly it also establishes the fact that local lipid gradients in a cell must exists sufficient to activate a channel. The putative concentration, local to the channel (grey cylinders), is indicated by an inset circle. PA, shown with black stick and red circle, is in concentrations low relative to a channel’s Kd. Localization of phospholipase D (PLD) and local production of PA results in a local high concentration of PA (>1 mM) and channel activation (gating). Local PA concentration is estimated from a single molecule of PA produced within 100 Å of the channel. Additional putative enzymes that could generate local PA include diacylglycerol kinase (DAGK) and lysophosphatidic acid acyltransferase (LPAAT). (B) Emerging class of PA regulated channels. At least 11 channels are known to respond directly to PA. Both nAChR and TRAAK require relatively high concentrations of PA suggestion local high concentrations may contribute to their activation [18,66]. Kv1.2/2.1 is a channel chimera [44]. TRESK is activated by a mixture of anionic lipids [71]. (C) PIP2 tonic concentration is high in the bulk membrane (~1–10 μM, red shading) [4] relative to a channels Kd (~200 nM), shown as black sticks with two red spheres. A hypothetical ion channel is shown with phospholipase C (PLC) bound to a disorder loop (black line). Local hydrolysis depletes PIP2 and decreases PIP2 binding resulting in a closed channel. Additional putative enzymes that could locally deplete PIP2 include phospholipase A2 (PLA2) and phosphoinositide 3 kinase (PI3K). Bulk PIP2 and PA concentrations are estimated from Kir and K2P lipid binding constants [18].