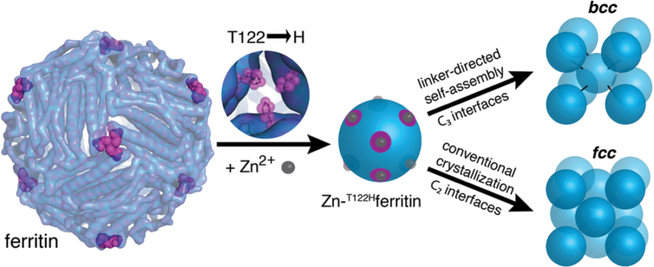
Fig. 27.
Schematic illustration for metal/linker-directed self-assembly of ferritin into three-dimensional crystals. Surface exposed Zn2+ binding sites are engineered at 3-fold symmetry (C3) sites of ferritin by mutating threonine 122 to histidine (T122H). In the presence of ditopic organic linkers, the T122H ferritin mutant forms a BCC lattice through coordination of ferritins at the C3 sites. On the other hand, in the absence of such ditopic linkers, the mutant forms a FCC lattice, which ferritin typically crystalized into, through coordination at 2-fold symmetry (C2) sites. Figure reproduced from ref. 377 with permission from the American Chemical Society, copyright 2015.