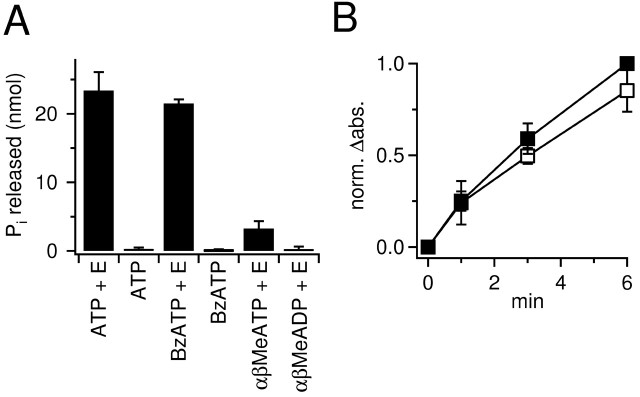
Figure 5.
BzATP is hydrolyzed in vitro by E-NTPDase and E-5NTase. A, Incubation of ATP, BzATP, α,β-MeATP, or α,β-Me-ADP with the enzymes in vitro resulted in accumulation of Pi. Note that an identical amount of Pi was released from ATP and BzATP. Without enzymes (E), only negligible amounts of Pi were detected. Incubation of α,β-MeATP yielded only a small amount of Pi. α,β-MeADP was resistant to hydrolysis. B, Time course of Pi release from ATP (▪) and BzATP (□) during the incubation of these adenine nucleotides with ecto-nucleotidases. The reactions were started in parallel and stopped successively after 1, 3, and 6 min. Enzymatic activity and Ca2+ concentration were strongly decreased to reduce the reaction rate. After 1 min, only a small portion of substrates has been hydrolyzed, and >6 min would be required under those conditions to achieve equilibrium. Note that the initial accumulation of Pi, being proportional to the initial reaction velocity, is similar for BzATP and ATP. Absorption values were normalized in each run on the absorption value of the ATP vial that was incubated for 6 min (norm.Δabs.).