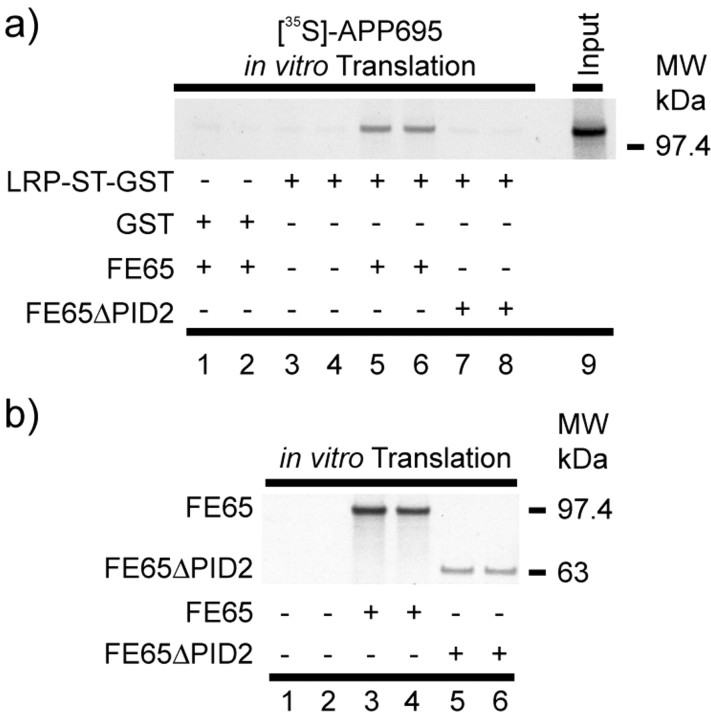
Figure 3.
LRP-ST-glutathione S-transferase pull-down of in vitro translated FE65-APP695 complex. a, APP695 was in vitro translated in the presence of [35S]-Methionine and incubated with in vitro translated FE65 (lanes 5, 6) or FE65ΔPID2 (lanes 7, 8). As control, [35S]-APP695 was incubated without FE65 or FE65ΔPID2 (lanes 3, 4). After incubation with LRP-ST-glutathione S-transferase fusion protein or GST fusion protein alone (lanes 1 and 2), bound proteins were coprecipitated by the addition of glutathione-agarose. Radiolabeled APP695 was detected by exposure to x-ray film, and the radioactive decays were quantified using phosphorimaging techniques. Note that only in the presence of functional FE65, ∼30% of the radiolabeled APP695 was pulled down by LRP-ST-glutathione S-transferase. The in vitro translated FE65 and FE65ΔPID2 proteins used in these experiments are shown in b. MW, Molecular weight.