Figure 2.
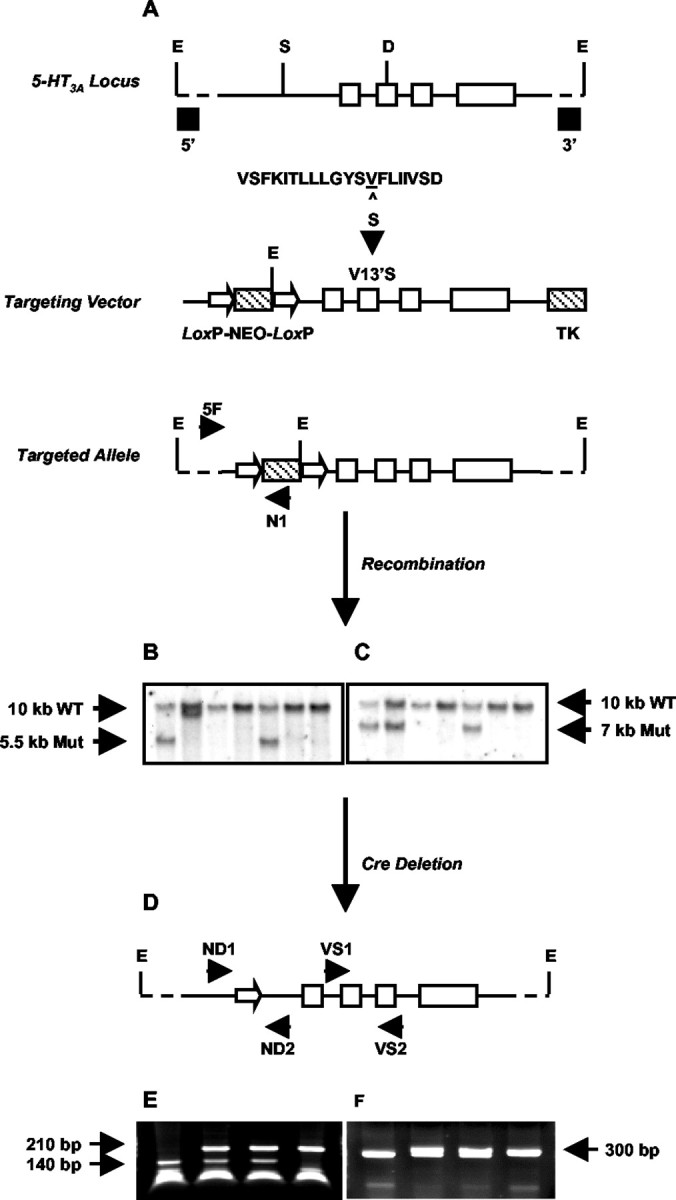
Strategy for generating 5-HT3A knock-in mice through targeted exon replacement. A, A 6 kb genomic fragment of the mouse 5-HT3A receptor gene (solid bar) was used to introduce a point mutation (V13′S) into exon (open boxes) 7 coding for the M2 domain of the 5-HT3A receptor. The sequence of the mouse 5-HT3A receptor M2 region (positions 1′ to 20′) is shown. The Val residue at 13′, corresponding to amino acid position 290 in the entire sequence, is underlined. In the 5-HT3A knock-in mouse this is mutated to Ser (S). Neo and TK selection markers (hatched boxes) were inserted into the SwaI site (S) in the large intron preceding exon 6 and at the 3′ end of the construct, respectively. The Neo sequence was flanked by two LoxP sites (open arrowheads). The linearized targeting vector was used for transfection of embryonic stem cells, and the resulting targeted allele was screened as described in Materials and Methods. B, C, Representative Southern blots of EcoRI (E)-digested genomic DNA were probed with either a 5′-flanking probe (B) or a 3′-flanking probe (C) to identify the wild-type and mutant (Mut) alleles. D, 5-HT3A-targeted mice were crossed to Cre-expressing transgenic mice as described in Materials and Methods, resulting in a Neo deleted mutant allele with one 34 bp LoxP site remaining in intron 5. E, F, Representative PCR screening of genomic DNA from Neo-deleted mice. E, Screening across the mutation site using primers VS1 and VS2 followed by DdeI (D) digestion. F, Screening across the single LoxP site after removal of the Neo cassette, using primers ND1 and ND2.
