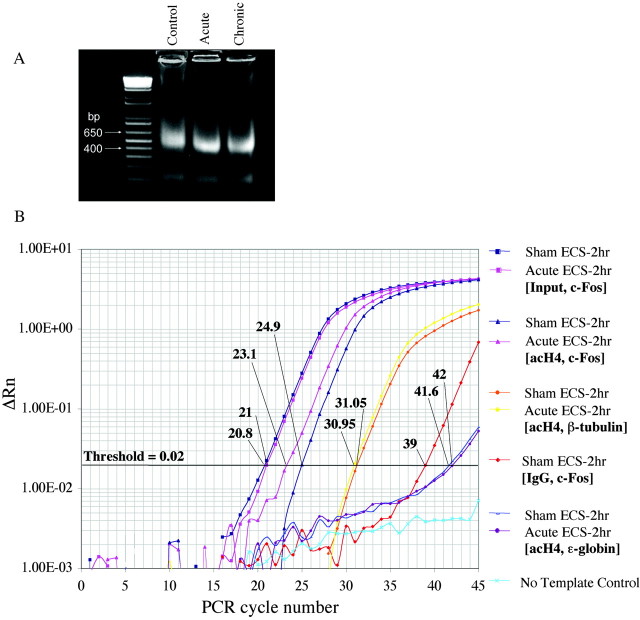
Figure 1.
Representative steps in the chromatin immunoprecipitation assay used to measure histone modifications in the hippocampus of ECS-treated and control rats. A, Chromatin was sonicated to fragments of 400-600 bp in size and run on a 2% agarose gel. Ethidium bromide staining of a resulting, representative gel is shown. B, Levels of acetylated H4 at various gene promoters in the hippocampus were quantified using real-time PCR, by comparing relative Ct values for the various genes of interest at a threshold of 0.02. (Parallel experiments, not shown, were performed for acetylated and phosphoacetylated H3.) Ct values of immunoprecipitated samples (Control and Acute ECS-2 hr with antibody specific for acetylated H4 and PCR amplified at the c-fos gene promoter) were normalized to Ct values obtained from “Input,” or non-immunoprecipitated genomic DNA, at which there is no difference between acute ECS and control samples, as expected. Comparison of these Ct values revealed a 3.5-fold increase in levels of H4 acetylation at the c-fos gene in acute ECS versus control (Sham ECS) samples. In aliquots of the same samples, Ct values from theβ-tubulin gene differed by a negligible 0.1, reflecting no regulation by acute ECS. Immunoprecipitation with nonimmune IgG yielded much higher Ct values, typically 16 cycles higher, indicating a ∼65,000-fold difference (i.e., negligible precipitation of the c-fos gene in the absence of specific antibody). Finally, analysis of ϵ-globin, a gene that is silenced in adult animals and contained within transcriptionally inactive heterochromatin, reveals virtually nondetectable levels of H4 acetylation.