Figure 3.
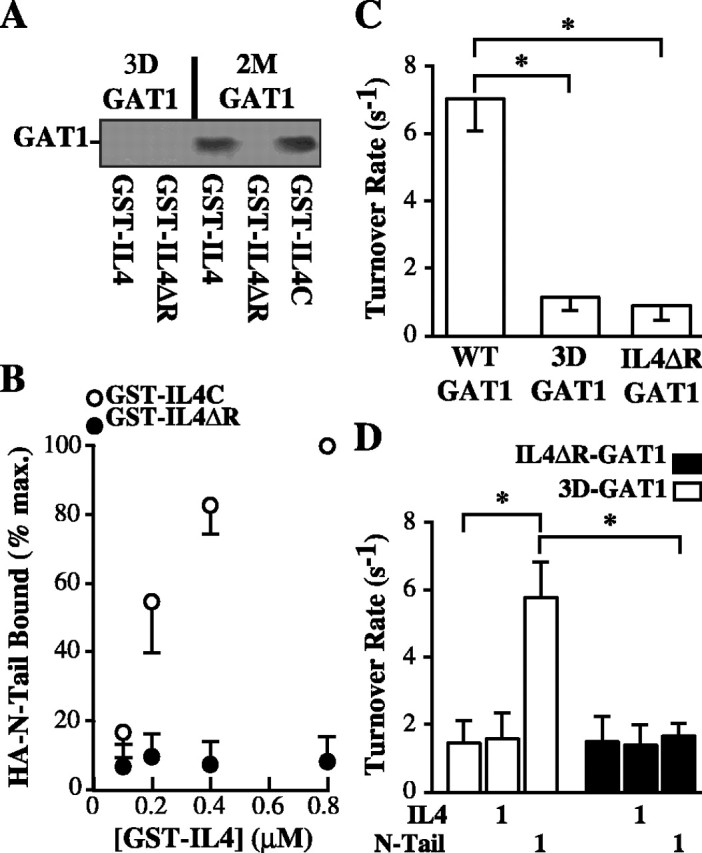
Charged amino acid residues in the N-terminal and IL4 domains are important for the functional interaction. A, Mutations in the N-terminal or IL4 domains eliminate the physical interaction. Cell lysates from oocytes expressing a GAT1 construct lacking three aspartic acid residues in the N-terminal tail (3D-GAT1) or a control mutant (2M-GAT1) were subjected to pull-down assays using GST constructs containing wild-type IL4 domain (GST-IL4), IL4 domain lacking three arginine residues (GST-IL4ΔR), or a control IL4 domain mutant (GST-IL4C). Bound proteins were subjected to immunoblotting using GAT1 Ab. Data are representative of three experiments. B, Mutation of arginine residues in the IL4 domain eliminates N-terminal tail binding. Recombinant GAT1 HA-N-Tail cytosolic domain was bound to increasing concentrations of GST-IL4C (open circles) or GST-IL4ΔR (filled circles), precipitated, and immunoblotted with an HA-specific Ab. Data are from three experiments plotted relative to binding with 0.8 μm GST-IL4C fusion protein. C, N-terminal and IL4 mutant GAT1 constructs show decreased turnover rates. Oocytes expressing wild-type GAT1, 3D-GAT1, or IL4ΔR-GAT1 were subjected to two-electrode voltage clamp. Data are from 8-10 oocytes per condition. D, N-Tail fusion protein does not rescue the turnover rate of IL4ΔR-GAT1. Oocytes expressing 3D-GAT1 (open bars) or IL4ΔR-GAT1 (filled bars) were left untreated or acutely injected with 1 μm IL4 or N-Tail fusion protein and then subjected to two-electrode voltage clamp. Data are from 6-11 oocytes per condition. Asterisks indicate significant differences (p < 0.05) between the two indicated groups.
