Figure 2.
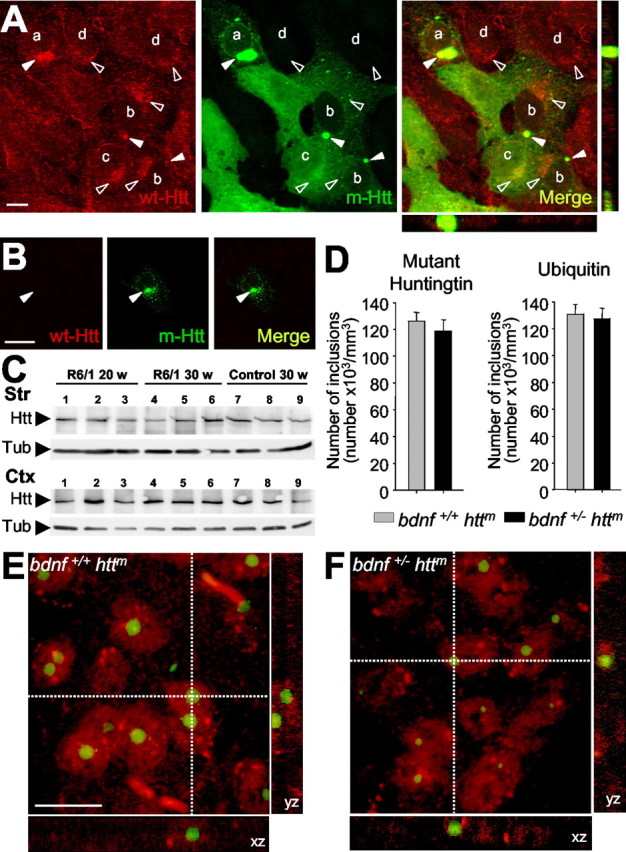
Sequestering wt Htt downregulates BDNF. A, Within nontransfected neural stem cells derived from the striatum (M213; d), wt Htt localizes in endocytic structures (open arrowheads). This localization is also detected in cells transfected with the exon 1 of htt with 72 repeats (qp72) that do not present aggregates (c) or that present small aggregates (b). However, in cells that express the qp72 construct and present large aggregates (a), wt Htt is only detected into the aggregates (filled arrowheads). We used the monoclonal antibody Mab2166 (Chemicon) to detect wt Htt (red; wt-Htt), whereas the mutant Htt was detected by the endogenous EGFP of the fusion protein (green; m-Htt). B, No signal for wt Htt was detected in the aggregates (filled arrowheads) in experiments avoiding the primary antibody. C, Western blot showing the levels of wt Htt in the cerebral cortex (Ctx) and in the striatum (Str) from R6/1 animals at 20 weeks (lanes 1-3) or at 30 weeks (lanes 4-6) or from wild-type mice at 30 weeks (lanes 7-9). Note that there are no changes in the levels of wt Htt (Htt) in R6/1. The levels of α-tubulin (Tub) are shown as a loading control. D-F, The levels of BDNF in mutant htt mice do not modify the number or morphological aspects of the intranuclear inclusions. Animals with mutant htt had intranuclear inclusions, which can be detected by mutant Htt or ubiquitin immunohistochemistry. D, The same density of aggregates was detected in both immunostaining patterns per genotype. In addition, similar results are shown in both R6/1 mice (bdnf+/+ httm) and bDM (bdnf+/ - httm), without significant differences between the two groups. E, F, Double immunohistochemistry against NeuN (red) and ubiquitin (green) demonstrated the same intranuclear location in both genotypes, containing mutant htt, with normal levels of BDNF (R6/1 mice; E) or with lower bdnf expression (bDM; F). Scale bars: A, 10 μm; B, 15 μm; (in E) E, F, 10 μm.
