Figure 1.
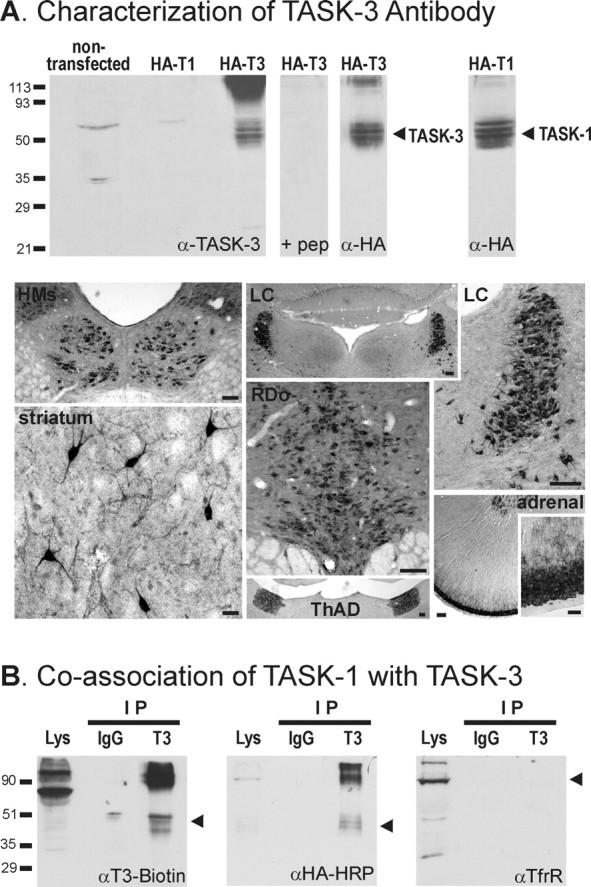
TASK-1 coimmunoprecipitates with TASK-3 in HEK 293 cells. A, Characterization of TASK-3 antibody. Top, Lanes were loaded with 5 μg of membrane preparations from HEK 293 cells transfected with the indicated constructs, and proteins were separated by 10% SDS-PAGE. Epitope-tagged HA-TASK-3 (T3) was identified with the anti-TASK-3 antibody; no immunoreactivity was seen for the corresponding HA-TASK-1 (T1) channel construct, or for HA-TASK-3 when the antibody was preadsorbed with excess antigenic peptide (pep). Essentially identical bands were recognized with an antibody to the epitope (α-HA), which was also used to confirm expression of HA-TASK-1. Bottom, Immunohistochemistry was used to detect TASK-3 distribution in sections of rat brain and adrenal gland. TASK-3-like immunoreactivity was detected in a distribution pattern that was strikingly similar to previous TASK-3 in situ hybridization reports (Karschin et al., 2001; Talley et al., 2001; Vega-Saenz de Miera et al., 2001; Bayliss et al., 2003). Especially strong labeling was seen in cell bodies from the following regions (clockwise from top left): hypoglossal motor nucleus (HMs), locus ceruleus (LC), adrenal gland, anterodorsal thalamic nucleus (ThAD), dorsal raphe nucleus (RDo), and striatum. Scale bars: striatum, adrenal inset, 50 μm; others, 100 μm. B, HEK 293 cells were cotransfected with TASK-3 and HA-TASK-1 constructs. The TASK-3 antibody coimmunoprecipitated (IP) TASK-3 (left) and HA-TASK-1 (middle) but did not immunoprecipitate transferrin receptor (TfrR), an endogenously expressed membrane protein (right); a control nonspecific rabbit IgG did not immunoprecipitate either TASK subunit. Molecular weight markers (kilodaltons) are indicated on the left; arrowheads indicate locations of precipitated proteins.
