Figure 6.
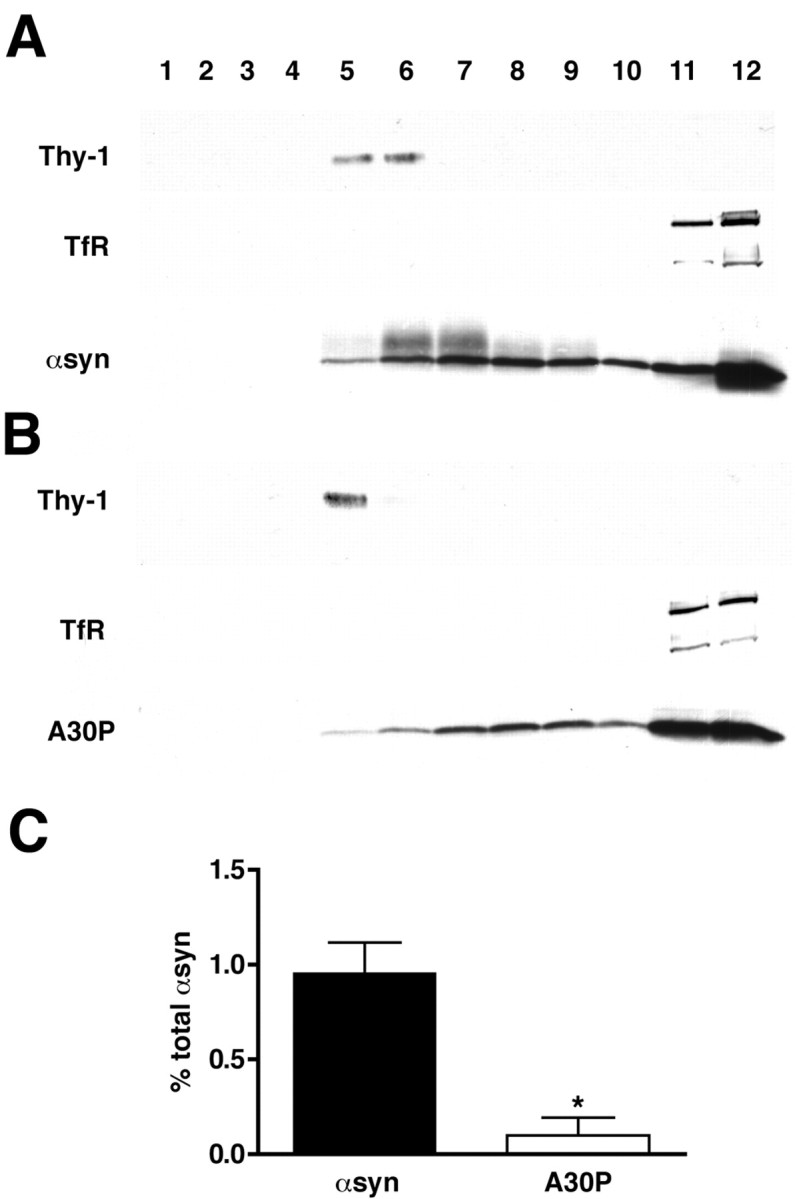
α-Synuclein associates with detergent-resistant membranes in mouse brain. Synaptosomes were isolated from the cortices of transgenic mice overexpressing human wild-type (A) and A30P (B) α-synuclein, solubilized in TX-100 and fractionated as described in Figure 3. A proportion of wild-type α-synuclein comigrates with Thy-1, whereas transferrin receptor (TfR) remains at the bottom of the gradient. Fractions were collected and numbered from the top of the gradient. α-Synuclein immunoreactivity in Thy-1-positive fractions was quantified by densitometry and expressed as a percentage of total immunoreactivity in all fractions. Quantification of the binding from three independent experiments (C) shows a clear reduction in binding for the A30P mutant relative to wild-type α-syn. Bar represents averages of three mice ± SD. *p = 0.0014; Student's t test.
