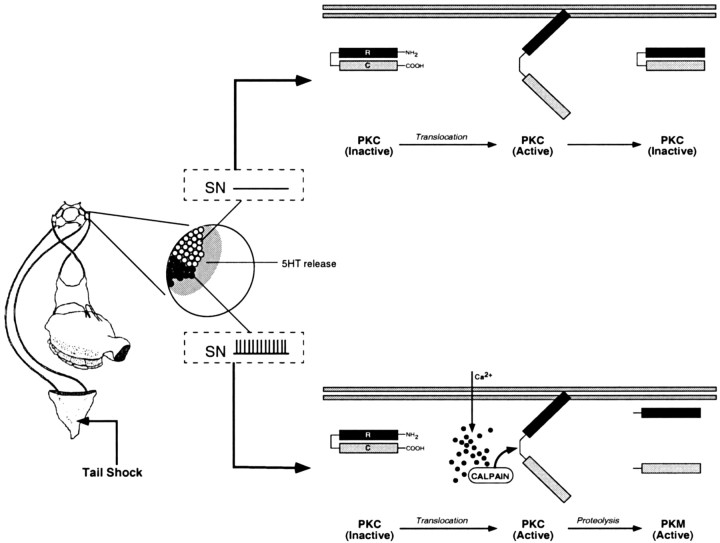
Figure 8.
A model for the induction and maintenance of site-specific ITM. A schematic representation of the proposed molecular events related to PKC activation in tail SNs (or possibly interneurons activated by these SNs) after tail shock is shown. Under basal conditions, PKC is retained in the cytosol, and its catalytic activity is inhibited by the N-terminal regulatory domain (R) that acts as a pseudosubstrate for the C-terminal catalytic domain (C). A single tail shock, regardless of location, will elicit 5-HT release (shaded area) that is distributed across the entire population of SNs and their synapses (Marinesco and Carew, 2002). 5-HT, acting through phospholipase C-coupled receptors (Li et al., 1995), stimulates translocation of PKC to the membrane, inducing a conformational change that relieves the autoinhibition of PKC activity by the regulatory domain. In SNs with receptive fields that lie outside the region of the tail receiving shock (open circles and top), 5-HT-induced PKC activation will be transient, enduring only as long as the short-term modulatory effects of 5-HT on second messengers persist. Conversely, the SNs innervating the shocked site will be activated (Walters et al., 1983a,b) coincident with 5-HT exposure (filled circles and bottom). SN activation increases intracellular Ca2+ influx, stimulating the Ca2+-activated protease calpain. Calpain, in turn, proteolyses activated PKC, generating persistently active PKMs. Thus, the induction of site-specific sensitization entails the proteolytic processing of activated PKC, the product of which (PKM) serves to maintain the memory in the intermediate-term temporal domain.