Figure 1.
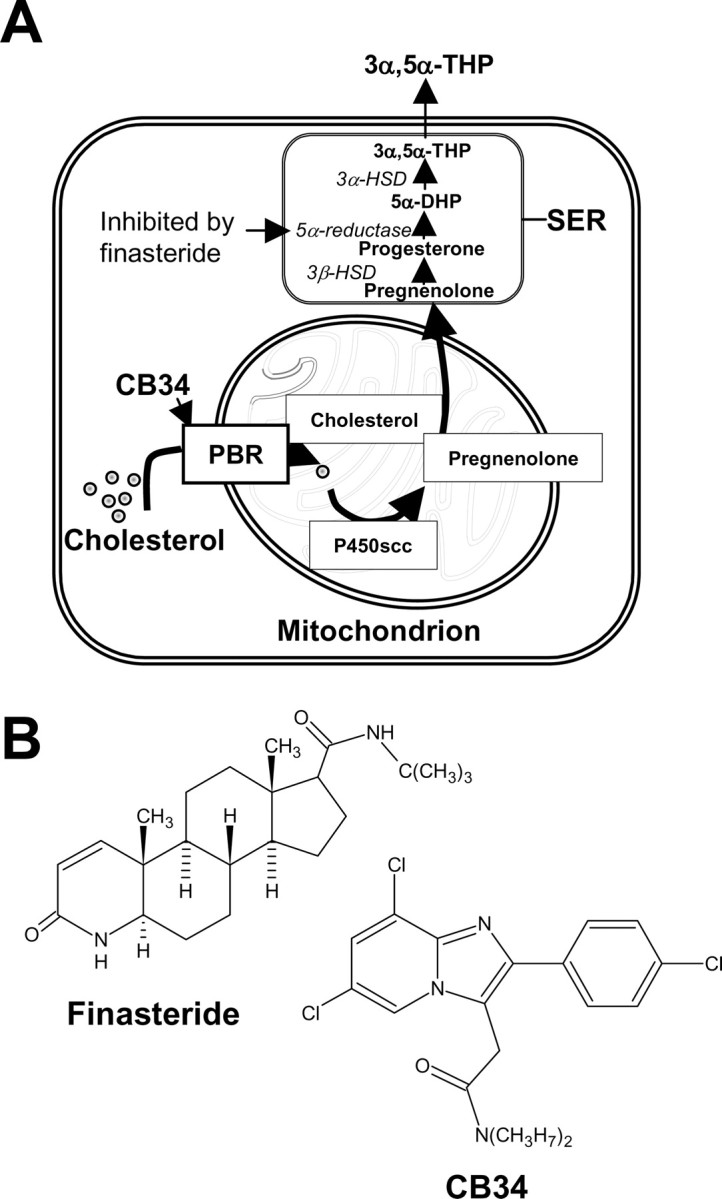
A, Pathway in glial cells of the hippocampus for the biosynthesis of the neurosteroid 3α,5α-THP (3α-hydroxy-5α-pregnan-20-one or allopregnanolone) from cholesterol. Translocation of cholesterol on the inner part of mitochondria, the first rate-limiting step in neurosteroid synthesis, is performed by several proteins, among others, the PBR, which in our experimental protocol was activated by the selective agonist CB34 (Serra et al., 1999). Finasteride, also used in our experiments, inhibits the enzyme 5α-reductase, thus blocking the conversion of progesterone to 5α-DHP and the formation of 3α,5α-THP. P450scc, Mitochondrial cholesterol side-chain cleavage enzyme; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 5α-DHP, 5α-dihydroprogesterone; 3α-HSD, 3α-hydroxysteroid dehydrogenase; SER, smooth endoplasmic reticulum. B, Chemical structure of finasteride and CB34.
