Figure 4.
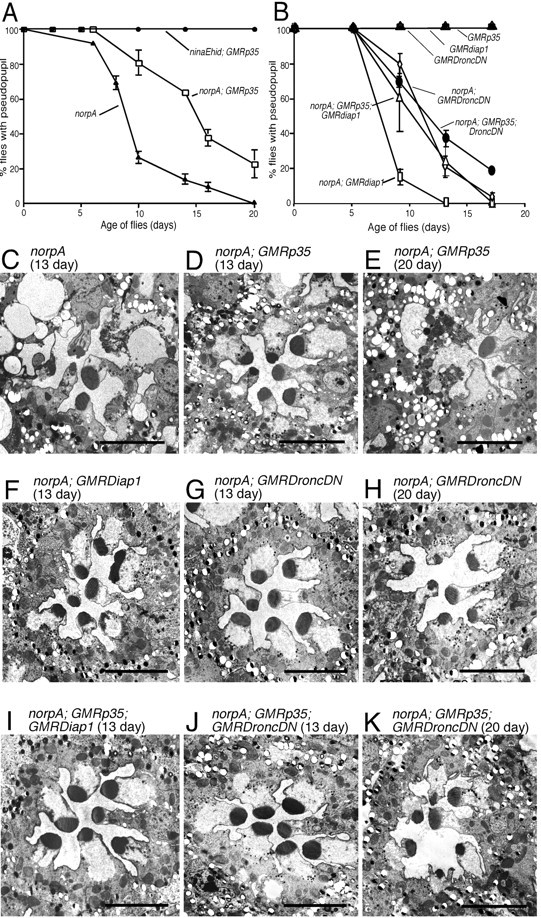
P35, Dronc, and Diap suppression of norpA in a low temperature environment. All flies were reared at 18°C under constant light of 2700 lux. A, Rate of retinal degeneration assessed by DPP analysis of norpA and norpA; GMRp35. GMRp35 delayed the loss of the DPP in norpA by ∼5 d. Under these conditions, and all others tested, the same GMRp35 transgene provided complete suppression of ninaEhid-triggered degeneration. B, Rate of norpA retinal degeneration assessed by DPP analysis in the presence of DroncDN, DroncDN, and P35, Diap1, and Diap1 and P35 cell death suppressors. Neither DroncDN nor Diap1 enhanced P35 suppression. C-E, Electron micrographs of photoreceptors from norpA and norpA; GMRp35 flies at 13 d (C, D) and norpA; GMRp35 flies at 20 d (E) from the same experiment shown in A. Consistent with the DPP analysis and previous experiments, GMRp35 provides some suppression at the earlier time point, with more extensive degeneration evident by 20 d. F-H, Electron micrographs of photoreceptors from norpA; GMRDroncDN and norpA; GMRdiap1 flies at 13 d (F, G) and norpA; GMRDroncDN flies at 20 d (H) from the same experiment shown in B. Consistent with the DPP analysis, initial signs of degeneration are seen at 13 d, with more extensive degeneration evident at 20 d (data not shown for GMRdiap1). I-K, Electron micrographs of norpA photoreceptors expressing P35 with either DroncDN or Diap1 at 13 d (I, J) and P35; DroncDN at 20 d. Consistent with the DPP analysis, no improvement in retinal structure is provided by simultaneous expression of two different caspase inhibitors. Scale bars, ∼5 μm.
