Figure 4.
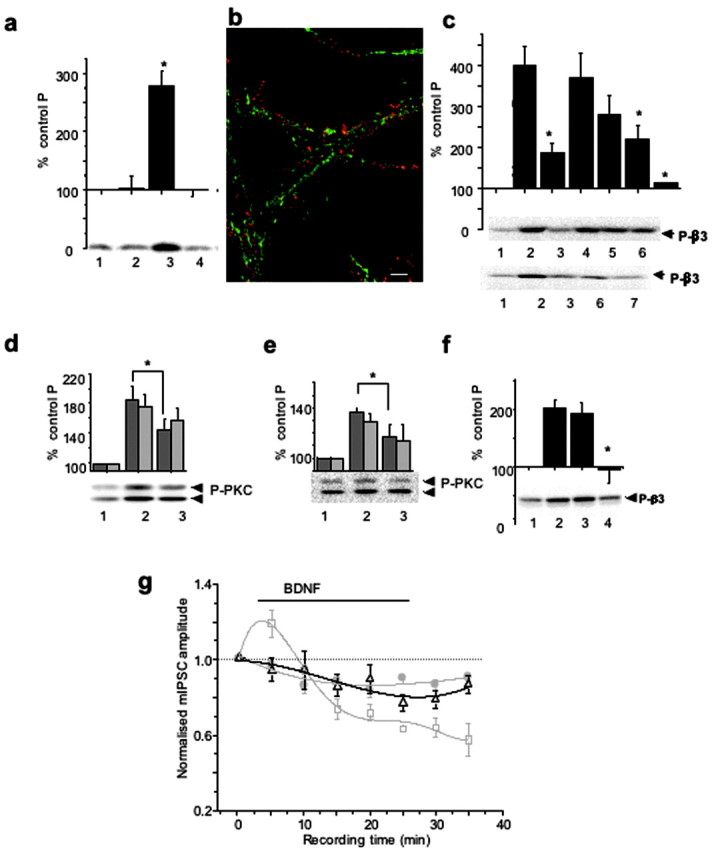
PKC activity mediates both BDNF-dependent GABAAR phosphorylation and potentiation of mIPSCs. a, K252a-inhibited BDNF-induced phosphorylation of GABAAR-β3. Cortical neurons were incubated alone (1), with 200 nΜ K252a (2), with 100 ng/ml BDNF (3), or with 200 nΜ K252a + 100 ng/ml BDNF (4) for 10 min and then lysed. Immunoblotting with anti-P-β3 antibody was performed using equal protein amounts of SDS lysates. *p < 0.05 (n = 3). b, Subcellular localization of TrkB (red) and GABAAR-β3-subunit (green) in hippocampal neurons (14 DIV). Scale bar, 10 μm. c, Potentiation of GABAAR-β3 phosphorylation by BDNF is dependent on PKC and PI-3 kinase activities. Cortical neurons were incubated alone (1), or with 0.2 μm calphostin C (3); 200 μm Rp-8Br-cAMP (4); 2 μm KN-93 (5); 2 μm LY 294002 (6); or both calphostin C and LY 294002 (lane 7) for 10 min, followed by the addition of BDNF (100 ng/ml), and incubated for an additional 10 min. Lane 2 represents samples treated with BDNF alone. Immunoblotting with anti-P-β3 antibody was then performed as in a. The panels below the histogram show individual examples of immunoblots. BDNF-induced β3-subunit phosphorylation was significantly inhibited in the presence of calphostin C, LY294002, or both inhibitors. *p < 0.05 (n = 4-7). d, e, BDNF-enhanced phosphorylation of PKC on Ser660 was dependent on PI-3 kinase activity (d) or TrkB kinase activity (e) in cortical neurons incubated in the absence (1) or presence of either 2 μm LY294002 (d, lane 3) or 200 nm K252a (e, lane 3) for 10-15 min, followed by the addition of BDNF (100 ng/ml) and additional incubation for 10 min (lane 2). Equal protein amounts were subjected to immunoblotting with pan-P-PKC antibody. *p < 0.05) (n = 4). The panel below the histogram shows a representative immunoblot of the higher molecular mass band migrating at 97 and the lower band migrating above 66 kDa. f, Potentiation of GABAAR-β3 phosphorylation by BDNF was abrogated by BAPTA-AM in cortical neurons. Cells were incubated in the absence (lanes 1 and 2) or presence of either 1 μm EGTA (lane 3) or 25 μm BAPTA-AM (lane 4) for 5-10 min, followed by the addition of BDNF (100 ng/ml) to samples in lanes 2-4. Incubation was then extended for an additional 10 min. Immunoblotting with anti-P-β3 antibody was performed using equal protein amounts of SDS lysates. *p < 0.05 (n = 4). The panel below the histogram shows a representative immunoblot. g, Time-stability relationships for hippocampal mIPSC amplitudes in control Krebs' solution (filled circles) and after the application of either 200 ng/ml BDNF (squares) or 200 nm calphostin C (triangles). BDNF was applied for the duration indicated by the solid line (see Materials and Methods), and calphostin C was applied to the Krebs' solution before the start of the recordings. The mIPSC amplitudes were normalized to the mean mIPSC amplitudes in control Krebs' solution. All points are means ± SE from n = 9 cells. Data for the control and the BDNF treatments were obtained from Figure 1a.
