Figure 6.
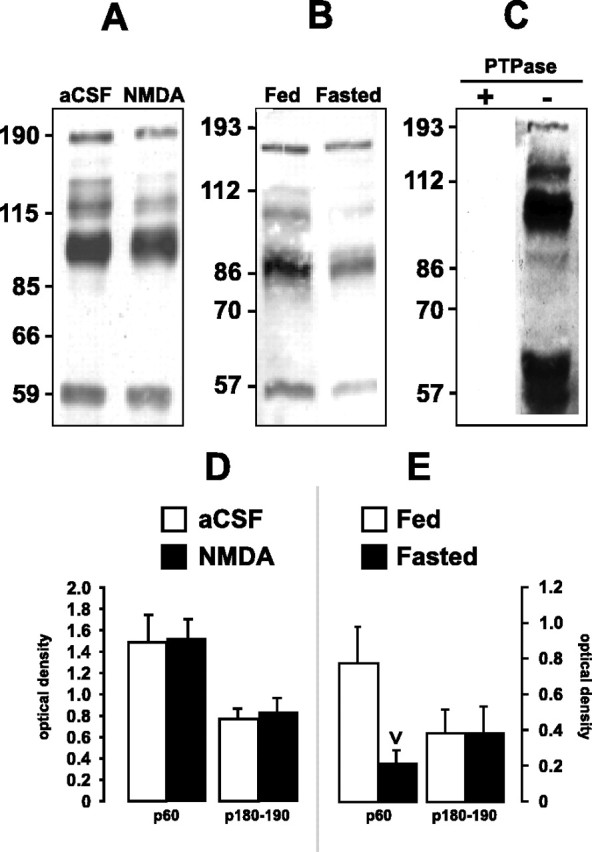
Tyrosine-phosphorylated proteins are present within total protein extracts of homogenized LHA-containing tissue. A, B, Total protein within LHA-containing tissue from aCSF- and NMDA-injected rats (A) or ad libitum-fed or 48 hr-fasted rats (B) was probed with an α-pY antibody. Positions of molecular weight standards are along the left of the blots (in kilodaltons). Note the prominent ∼60, ∼95, and ∼180-190 kDa bands. Data are representative of four experiments, each performed using tissue obtained from one or two animals. D, E, Tyr-P levels are expressed as OD units ± SEM for the 60 and 180-190 kDa bands in tissue from aCSF- and NMDA-injected rats (D) or ad libitum-fed and 48 hr-fasted rats (E). Compared with controls, fasted rats had significantly decreased Tyr-P for the 60 kDa band (inverted carat; p < 0.05). C, Specificity of the α-pY antibody. The α-pY immunoreactivity for total protein from LHA-containing tissue was abolished with (+) but not without (-) tyrosine phosphatase pretreatment.
