Figure 7.
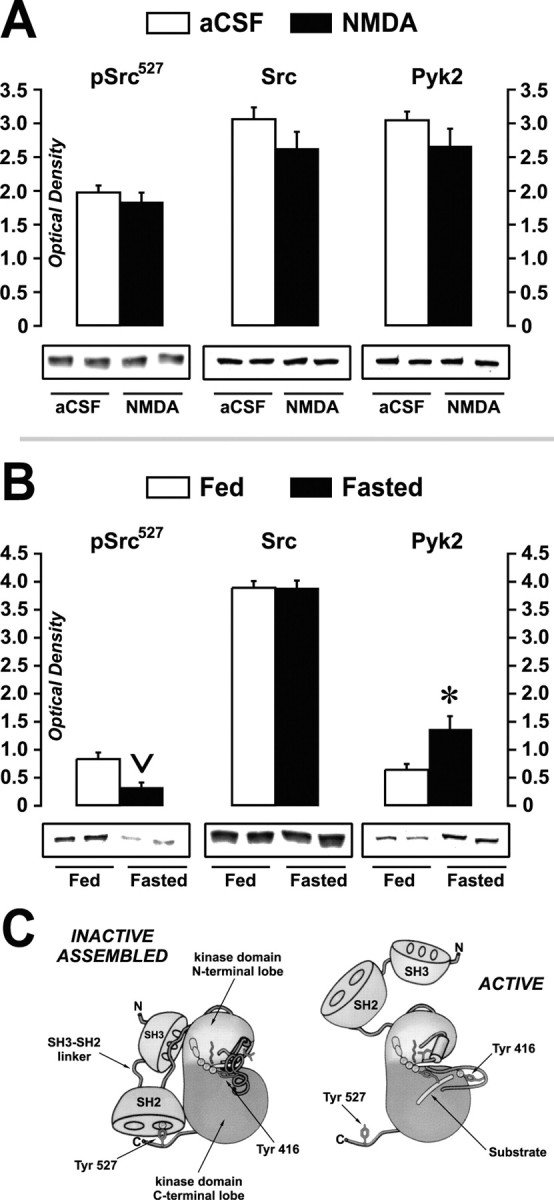
Apparent Src activity and Src and Pyk2 expression in LHA-containing tissue after NMDA injection or food deprivation. Immunoblots of LHA-containing tissue homogenates from aCSF- and NMDA-injected rats (A) or from 48 hr-food-deprived (Fasted) and ad libitum-fed (Fed) rats (B) are shown. Two lanes are shown for each treatment to help illustrate the uniformity of immunostaining within treatment groups. Blots were probed with antibodies directed against the negative regulatory phosphorylation site on Src family kinases at Tyr527 (pSrc527), Src kinase, or Pyk2 kinase, and immunostaining was quantitatively measured as mean optical density values (all ordinate scales) ± SEM. For each antigen, blots are representative of two or three measures. C, Illustration of inactive versus active Src kinase (modified and reproduced with permission from Young et al., 2001). In the INACTIVE ASSEMBLED conformation, note the hypothesized interaction of the phosphoryl group at Tyr527 with an Src homology 2 (SH2) domain binding site. Dephosphorylation at Tyr527 is thought to loosen an inducible snap lock produced by the SH2-SH3 linker domain, disinhibiting the kinase and facilitating greater exposure of the SH2 and SH3 domains to protein-protein binding interactions with other macromolecules (ACTIVE conformation). From this mechanism, the fasting-induced decrease in phosphorylation of Src at Tyr527 (B) suggests that Src is more catalytically active during food deprivation.
