Abstract
Corticotropin-releasing hormone (CRH) and GABA have been implicated in depression, and there is reason to believe that GABA may influence CRH functioning. The levels of CRH, and mRNA for CRH-binding protein, CRH1, and CRH2 receptors, as well as various GABAA receptor subunits (α1, α2, α3, α4, α5, δ, and γ2), were determined in several frontal cortical brain regions of depressed suicide victims and nondepressed individuals who had not died by suicide. Relative to the comparison group, CRH levels were elevated in frontopolar and dorsomedial prefrontal cortex, but not in the ventrolateral prefrontal cortex of suicide victims. Conversely, using quantitative PCR analyses, it was observed that, in frontopolar cortex, mRNA for CRH1, but not CRH2, receptors were reduced in suicide brains, possibly secondary to the high levels of CRH activity. In addition, mRNA of the α1, α3, α4, and δ receptor subunits was reduced in the frontopolar region of suicide victims. Interestingly, a partial analysis of the GABAA receptor functional genome revealed high cross-correlations between subunit expression in cortical regions of nondepressed individuals, suggesting a high degree of coordinated gene regulation. However, in suicide brains, this regulation was perturbed, independent of overall subunit abundance. These findings raise the possibility that the CRH and GABAA receptor subunit changes, or the disturbed coordination between these GABAA receptor subunits, contribute to depression and/or suicidality or are secondary to the illness/distress associated with it.
Keywords: cortex, CRF, depression, GABA, prefrontal, human suicide, QPCR
Introduction
Corticotropin-releasing hormone (CRH) has been implicated as a mediating factor in clinical depression (Nemeroff, 1996; Mitchell, 1998). Among other things, CRH mRNA expression and CRH immunoreactivity were increased in hypothalamic nuclei as well as the locus ceruleus and raphe nucleus of depressed suicides (Raadsheer et al., 1994, 1995; Austin et al., 2003; Bissette et al., 2003). In addition, elevated levels of the peptide were detected in CSF of depressed individuals (Nemeroff et al., 1984, 1991; Roy et al., 1987; Kling et al., 1991, 1994; Heuser et al., 1998; Arborelius et al., 1999; Wong et al., 2000).
Although frontal cortical disturbances may be involved in depression, relevant data concerning CRH status have been sparse and inconsistent. Although Nemeroff et al. (1988) found reduced CRH binding sites within the frontal cortex of depressed suicides, neither Hucks et al. (1997) nor Leake et al. (1990) observed such effects. Nonetheless, given the relationship between CRH and monoamine functioning (Ruggiero et al., 1999), the view was advanced that depression involves the disruption of the interface between CRH, norepinephrine (NE), and serotonin (5-HT) circuits (Wong et al., 2000). Moreover, these data have fostered attempts to treat major depression through pharmacological agents targeting CRH functioning (Holsboer, 2003; Nemeroff and Owens, 2003).
On the basis of increased plasma and CSF GABA levels, the effects of GABA-acting agents, and the influence of antidepressants on interneuronal functioning (Petty, 1995; Krystal et al., 2002; Brambilla et al., 2003), GABAergic processes have also been implicated in depressive illness (Lloyd et al., 1989; Shiah and Yatham, 1998; Brambilla et al., 2003; Tunnicliff and Malatynska, 2003). However, postmortem brain analyses have been less compelling. Although GABA levels within the frontal cortex were inversely related to severity of depression (Honig et al., 1988), others have reported that neither GABA levels (Korpi et al., 1988) nor GABA-related enzymes were altered in depressed suicides (Cheetham et al., 1988; Sherif et al., 1991). Interestingly, however, GABAA/benzodiazepine binding sites were elevated in depressed suicides (Cheetham et al., 1988).
CRH is uniquely expressed in GAD-positive interneurons in rat cortex, and chronic stressors can affect GABAA receptor expression (Yan et al., 1998; Cullinan and Wolfe, 2000). Likewise, GABAergic agents may affect CRH mRNA expression within limbic sites (Skelton et al., 2000; Stout et al., 2001; Gilmor et al., 2003). Thus, we assessed CRH peptide levels, and both CRH1 and CRH2 receptor mRNAs, because they may be differentially involved in depression. Finally, because the function of the GABAA receptor complex is primarily controlled by the expression of the α1 to -5, δ, and γ2 subunits, we assessed their expression and interrelationships in control and suicide brains. Given the important role for the prefrontal cortex in subserving various cognitive processes, including hedonia and behavioral planning (Kalivas and Nakamura, 1999; Tanji and Hoshi, 2001; O'Doherty et al., 2003), the receptor changes were assessed in several aspects of the frontal cortex, namely the frontopolar, dorsomedial, and ventrolateral prefrontal cortices.
Materials and Methods
Subjects
Brains from suicide victims (n = 12; 11 males and 1 female) and from control participants (n = 12; 6 males and 6 females) of approximately equal age (mean, 51.3 ± 4.00 and 45.0 ± 3.40, respectively; F(1,22) = 1.4; p = 0.241), who died suddenly from causes not directly involving any CNS diseases, were obtained at autopsy at the Department of Forensic Medicine of the Semmelweis University Medical School. The medical, psychiatric, and drug history of suicide victims was obtained by psychological autopsy. This included interviews with the attending physician or psychiatrist, family members, and relatives, as well as data obtained from medical or hospital records. In the case of all of the suicide victims, a psychiatric diagnosis of affective disorder was on record. These were done and/or confirmed by experienced psychiatrists on the basis of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria and included the following: major depressive disorder with recurrent episodes (n = 9); bipolar illness, depressed phase (n = 1); and major depression with psychotic features (n = 2). Those participants with a history of schizophrenia, epilepsy, alcohol, or other drug abuse were excluded. Suicide victims died by hanging (n = 7), drug overdose (n = 4), or jump from height (n = 1). As far as could be determined from psychological autopsy and medical records, the participants included in this study had not used antidepressant medication for at least 2 months before death and did not have a history of drug or alcohol abuse. Toxicological tests on blood samples did not reveal the presence of drugs or alcohol in cases of death by hanging or jump from height. Causes of death in control subjects were the following: acute cardiac failure (n = 6), myocardial infarction (n = 4), or traffic accident (n = 2). Examination of medical records of control subjects at the autopsy confirmed the absence of psychiatric illness, or alcohol or drug abuse during the last 10 years. All of the controls and suicide victims were Caucasian from Hungary (Budapest region). Harvesting of tissues was approved by the local ethics committee, and informed consent was obtained from next of kin.
Tissue collection, dissection, and storage
Brains were obtained 1-6 hr (mean ± SEM, 3.5 ± 0.46 and 2.75 ± 0.39 hr for suicide and controls; F(1,22) = 1.88; p = 0.18) after death. After removal from the skull, the brains were cut in six major pieces (four cortical lobes, basal ganglia-diencephalon, and lower brainstem-cerebellum), rapidly frozen on dry ice, and stored at -70°C until dissection (2 d to 2 months). At the time of the dissection, the brain samples were sliced into 1- to 1.5-mm-thick coronal sections at a temperature of 0-10°C. Three prefrontal cortical areas were cut out of the sections by a fine microdissecting (Graefe's) knife: (1) the frontopolar cortex (FPC) (Brodmann area 10), dissected at the most polar portion of the frontal lobe below the intermediate frontal sulcus, (2) the dorsomedial prefrontal cortex (DMPFC) (Brodmann area 9), taken just dorsal to the frontopolar area including the most polar portion of the superior and part of the middle superior gyrus between the superior and intermediate frontal sulci, and (3) the ventrolateral prefrontal (VLPFC) (Brodmann area 11), which includes a part of the inferior frontal gyrus and the lateral part of the orbitomedian zone. Cortical samples were always taken from the right hemisphere. The samples were stored in airtight containers or plastic tubes at -70°C until additional use. For assay purposes, the number of samples available varied across brain regions (FPC, 12 suicide, 12 control; DMPFC, 7 suicide, 11 control; and VLPFC, 6 suicide, 5 control).
Tissue analyses
CRH content. Tissue from each of the cortical regions (100-300 mg) was placed in microcentrifuge tubes containing 1 ml of 0.5 m acetic acid/1N HCl and heated to 80°C for 1 hr. The tissue was then sonicated (Kontes Micro-Ultrasonic Cell Disruptor; setting 5; Kontes, Vineland, NJ) and an aliquot frozen for protein determination, using a BCA Protein Analysis kit (Chromatographic Specialties, Brockville, Ontario, Canada) and a PC colorimeter (Brinkmann, Mississauga, Ontario, Canada). The remainder of the homogenate was centrifuged at 10,000 × g for 4 min, and the supernatant of each region was divided into three aliquots (300 μl), lyopholized, and stored at -20°C until subsequent peptide level determination.
A solid-phase RIA procedure was used for the detection and quantification of CRH (Merali et al., 1998). Briefly, well plates (Immunilon-4; Dynatec Laboratories, Chantilly, VA) were coated with protein A/G (Calbiochem, La Jolla, CA) at least 24 hr before use and stored at 0-4°C. On the day of the RIA, the plates were coated with CRH antibody (final dilution, 1:100,000). The standards (reconstituted in RIA buffer; ranging from 0.05 to 250 fmol for CRH), blanks, and samples were then pipetted into the designated wells and incubated for 24 hr at 0-4°C. Next, 5000-6000 cpm of 125I-Tyr0-rCRF (Amersham Biosciences, Oakville, Ontario, Canada), was added to each well and incubated for 24 hr at 0-4°C. Finally, the plates were rinsed, and the wells counted for residual radioactivity using a gamma counter (Cobra II Auto-Gamma, model D5002; Hoefer Scientific, Canberra Packard, Ontario, Canada).
The specific anti-CRH serum [rC70; kindly provided by W. Vale (The Salk Institute, La Jolla, CA)] recognizes CRF1-41 and cross-reacts poorly with other related peptides including urotensin 1 and urocortin (Vale et al., 1983).
Verification for authentic CRH. To verify the identity of the CRH-immunoreactive material detected in cortical tissue, the lyophilized samples were pooled (to enhance peptide content), and then subjected to separation by a reverse-phase HPLC system that consisted of a Spectra-Physics (San Jose, CA) P-2000 gradient pump and a C-18 Vydac (Hesperia, CA) column (model 218TP54; 250 × 4.6 mm, 5 μm; 300 Å pore size). The column was equilibrated with mobile phase A [10% acetonitrile (AcN) with 0.1% trifluoroacetic acid (TFA), in H2O], and peptides were eluted with mobile phase B (90% AcN with 0.1% TFA, in H2O), using a gradient (from A = 100% to B = 100%, over 95 min), at a flow rate of 1 ml/min. Authentic CRH standard (rat/human CRH1-41; Peninsula Laboratories, San Carlos, CA) was run under identical conditions, and the absorbance of the eluant was monitored at 214 nm (absorbance detector model 783A; Applied Biosystems, Foster City, CA). The peak elution time for synthetic CRH was 65.8 min, and that of CRH-immunoreactive material contained within the brain extracts eluted in fractions collected over 65-67 min, peaking at 66 min, coinciding with the elution time of authentic CRH.
CRH receptors, CRH-binding protein mRNA, and GABAA receptor subunit mRNA: reverse transcription-quantitative PCR analysis. Total brain RNA was isolated and purified by standard methodologies using Triasol according to the manufacturer's protocol. Isolated RNA was checked for purity by ensuring that the OD260/280 ratio was >1.8. Samples for quantitative PCR (QPCR) analyses were prepared by reverse transcribing 5.0 μg of total RNA using Superscript II reverse transcriptase (Invitrogen, Burlington, Ontario, Canada). Aliquots of this reaction were then used in simultaneous QPCR reactions.
For QPCR, SYBR green detection was used according to the manufacturer's protocol (Brilliant QPCR kit; Stratagene, La Jolla, CA). A Stratagene MX-4000 real-time thermocycler was used to collect the data. All of the PCR primer pairs used generated amplicons between 90 and 110 bp. Amplicon identity was checked by restriction analysis. Primer efficiency was measured from the slope relation between absolute copy number or RNA quantity and the cycle threshold using the MX-4000 software. All of the primer pairs had a minimum of 93% efficiency.
Primer pairs were as follows: synaptophysin, forward, CAGACAGGGAACACATGCAAGG, reverse, GGCCCAGCCTGTCTCCTTAAAC; CRH1, forward, TGGCCAGCAACATCTCAGG, reverse, CTCCCGGTAGCCATTGTTTG; CRH2, forward, TGCTTCTTCAATGGAGAGGTGC, reverse, GCCGTCTGCTTGATGCTGTG; CRH-binding protein, forward, AGCAGGTTGCGAGGGAATAGG, reverse, GCGCACCACAGTGTTGTCACAG; GABAA subunit α1, forward, ATCACAGAGGATGGCACCTTGC, reverse, TGGGCATCCATAGGGAAGTCC; α2, forward, AGAACAACGCTTATGCAGTGGC, reverse, GTGGTTGCACTCTTGGAGATGG; α3, forward, AACCGGGAGTCAGCTATCAAGG, reverse, TTGGGTGCCTGTATGCTTC; α4, forward, GGTTTCTGCCAAGAAGGTACCC; reverse, TTTAAACAAACCGCCAGGCAC; α5, forward, ACCAACGGCTCCACCAAGTC, reverse, TTCTCAGTGCCCACCGTCTG; δ, forward, GTCTCTAGGCATCACCACGGTG, reverse, TAGACGTCCAGTGCCTTGATGG; and γ2, forward, CTGGATCACCACTCCCAACAGG, reverse, GGCAGGAGTGTTCATCCATTGG.
Primers that amplify synaptophysin mRNA were used as a control to normalize the data. This mRNA species has been shown previously, even under extreme perturbations (static epilepsy), to be a stably expressed house-keeping gene (Chen et al., 2001). In addition, a second house-keeping gene, cyclophylin was used to confirm that differences in the CRH receptors or GABAA subunits were, in fact, not related to nonspecific factors. Although there was intersubject variability in the cycle threshold (Ct) for synaptophysin, there was no significant difference in the average Ct for each group (control, FPC, 22.4 ± 0.8; DMPFC, 21.4 ± 0.4; and VLPFC, 21.4 ± 0.5; suicide, FPC, 23.1 ± 1.0; DMPFC, 22.0 ± 0.7; and VLPFC, 23.1 ± 1.0; values of p > 0.20). Moreover, the subject-to-subject variability of the synaptophysin Ct values was unrelated to the postmortem harvest time (r = 0.14). To compensate for interindividual variability that ordinarily exists within the assay, the expression of each species was normalized by subtracting its Ct from the synaptophysin Ct. The normalized Ct values (denoted as Ctn) for each mRNA species were averaged for each brain region in both control and suicide groups. Because synaptophysin is a low-abundance mRNA species compared with most neurotransmitter receptor transcripts, the Ctn is usually positive. An average Ctn that is less than the Ctn of another comparison group indicates a relatively lower abundance of that mRNA species and vice versa, and a negative Ctn means that the mRNA species was less abundant than synaptophysin. The difference between Ctn values for an mRNA species represents the fold change (as power of 2) in abundance. A difference of 1 cycle represents doubling, 2 is quadrupling, and so on. Although the changes in some species was <1 cycle, where statistically significant these differences were still larger than the SE of the average Ctn.
For the QPCR analysis, aliquots of the same reverse-transcription reaction were used and set up from the same PCR reaction master mix run in parallel. In general, the QPCR analysis for the CRH receptor mRNA species were run together, and the GABAA receptor mRNA species were run together. Thus, a panel of mRNA species was always run for each subject simultaneously.
Statistical analyses
Data were analyzed by ANOVA independently for each brain region and CRH or receptor subtype. In cases in which correlations were conducted to assess the relationship between outcome variables, or between dependent and independent variables (e.g., age, time to autopsy), these were done by Pearson product moment correlation. In all of the cases, the α was set at 0.05. In two instances, the CRH values obtained were >5 SDs from the mean, and these data were excluded from the analysis, and in one control case, an extreme α3 score was excluded.
To assess the interrelationships between CRH1 receptors, CRH2 receptors, CRH-binding protein, and the various GABAA receptor subunits, cross-correlations were computed using Pearson product moment correlations. Because a priori predictions were not made concerning GABAA interrelationships, the α was set at 0.05 using a two-tailed test.
Results
Analyses of the CRH levels, as well as the CRH receptor, binding protein, and GABAA receptor subunit mRNA, indicated that their levels of expression were unrelated to the time required to harvest the brain samples. Likewise, control and suicide samples did not differ with respect to synaptophysin mRNA expression (mean ± SEM, FPC, 22.45 ± 0.83 and 23.10 ± 0.97; DPFC cortex, 21.40 ± 0.40 and 22.02 ± 0.25; VLPFC, 21.39 ± 0.24 and 21.78 ± 0.18, respectively) or with respect to that of cyclophylin (mean ± SEM, FPC, 27.31 ± 0.69 and 28.16 ± 0.87; DMPFC, 25.32 ± 0.47 and 25.91 ± 1.12; VLPFC, 27.06 ± 0.19 and 27.86 ± 0.54, respectively). Although the age of the suicide victims and the comparison group did not differ significantly, because the suicide victims were somewhat older, correlational analyses were performed to determine whether age was related to CRH levels and CRH mRNA receptor and GABAA subunit mRNA expression. These analyses indicated that, in each of the regions assayed, age was not correlated with peptide or receptor mRNA levels, with the exception of the α5 subunit, in which mRNA expression was inversely related to age in suicide victims, and the δ subunit in this region, in which mRNA expression was inversely related to age in the control brains. Finally, given that only one female brain was represented in the suicide condition, the influence of the sex variable could not be assessed. Moreover, because of the limited number of participants, and hence low degrees of freedom, analysis of covariance would not provide meaningful findings.
Frontopolar cortex
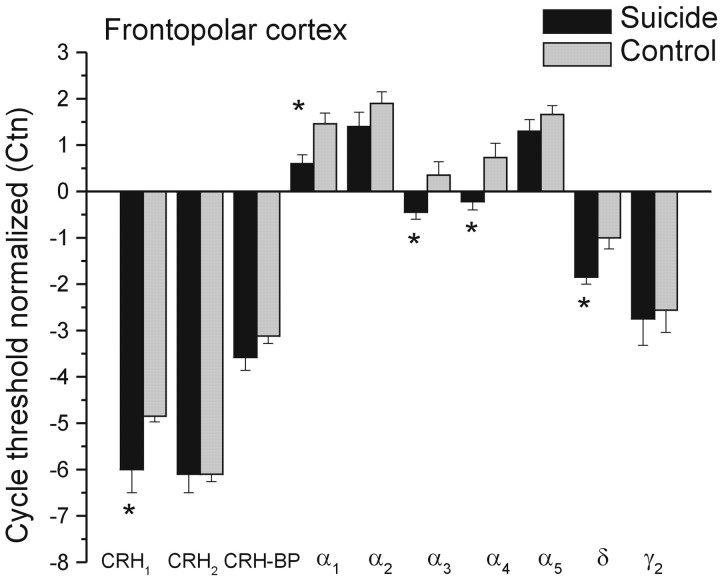
The level of CRH was significantly higher among suicide victims than in the control group (mean ± SEM, 227.52 ± 30.25 vs 147.46 ± 16.89 pg/mg of protein, respectively; F(1,20) = 5.83; p < 0.05). This relatively high CRH abundance was opposite to the CRH1 receptor mRNA expression, which was lower in the suicide than the control samples (Fig. 1). This difference in Ctn of 1.1 cycles corresponds to a ∼2.1-fold change, so in suicide brain, there was ∼55% less mRNA than in controls (F(1,22) = 4.76; p < 0.05). In contrast, the CRH2 receptor mRNA expression was comparable in the two groups (F < 1) as was the CRH-binding protein (F(1,22) = 2.09; p = 0.16).
Figure 1.
Mean (±SEM) mRNA expression of CRH1, CRH2, and CRH-binding protein (CRH-BP), as well as mRNA expression of GABAA subunits in the frontopolar cortex of depressed suicides and controls. Data are presented as normalized cycle thresholds (Ctn), wherein the expression of each species was normalized by subtracting its cycle threshold (Ct) from the synaptophysin Ct. Thus, a negative Ctn indicates that the mRNA species was less abundant than that of synaptophysin. A change of 1 Ctn is equivalent to a twofold difference in the abundance of that species. * p < 0.05 relative to control samples.
Analysis of the GABAA receptor mRNA expression indicated that the normalized cycle thresholds for many of the α subunit mRNAs were lower in the suicide than in the control FPC samples (Fig. 1). In particular, α1 subunit expression was elevated relative to the synaptophysin, but this difference was less among the suicide victims. Here, we found a Ctn difference of 0.9 cycles corresponding to ∼45% decrease in mRNA abundance (F(1,22) = 8.56; p = 0.007). Likewise, the expression of the α3 and α4 receptor subunits was significantly diminished in the suicide brains relative to that seen in the control brains (F(1,21) = 5.46 and 6.86; p = 0.029 and 0.016, respectively). Also noteworthy is that these levels were lower than synaptophysin, indicating low levels of expression. Similarly, the expression of δ mRNA subunits was lower in the suicide than the control sample (∼45%) (F(1,22) = 8.63; p = 0.007), and once again, their expression was low relative to synaptophysin. In contrast, the expression of α2, α5, and γ2 subunit mRNAs were not significantly different in the suicide and control brains.
Dorsomedial prefrontal cortex
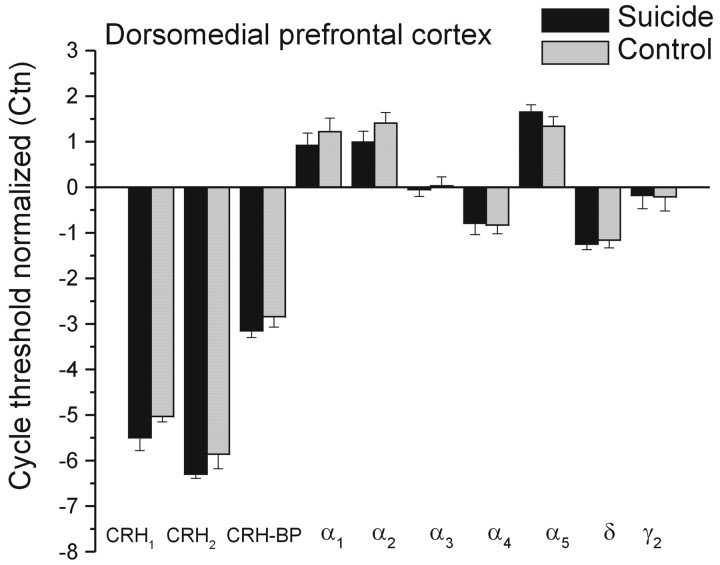
As in the case of the frontopolar cortex, CRH levels were elevated in the DMPFC of suicide brains relative to those of controls (mean ± SEM, 199.88 ± 39.95 vs 117.91 ± 16.56 pg/mg of protein; F(1,16) = 2.27; p < 0.05). There was a modest reduction of CRH1 mRNA expression in the suicide brains, but this difference was just shy of statistical significance (F(1,16) = 3.83; p = 0.068). Neither the expression of CRH2 nor that of the CRH-binding protein differed between the suicide and control brains (F(1,16) = 1.19 and 0.95; values of p > 0.10) (Fig. 2). As indicated previously, the CRH levels and the CRH1 mRNA expression were unrelated to the age of the individual or the time to postmortem analysis.
Figure 2.
Mean (±SEM) mRNA expression of CRH1, CRH2, and CRH-binding protein (CRH-BP), as well as mRNA expression of GABAA subunits in the dorsomedial prefrontal cortex of depressed suicides and controls. * p < 0.05 relative to control samples.
Unlike the mRNA expression of the GABAA subunits within the FPC, within the DMPFC, none of the mRNA expressions of the GABAA receptor subunits was found to differ between the groups. Although the number of brains assayed was relatively small, hence limiting the power to detect significant effects, it is unlikely that a true difference was missed, because the differences in Ctn between groups was typically <0.2 cycles and never >0.3 cycles. Thus, the absence of any meaningful differences between GABAA receptors does not seem to be a reflection of the small number of samples assayed.
Ventrolateral prefrontal cortex
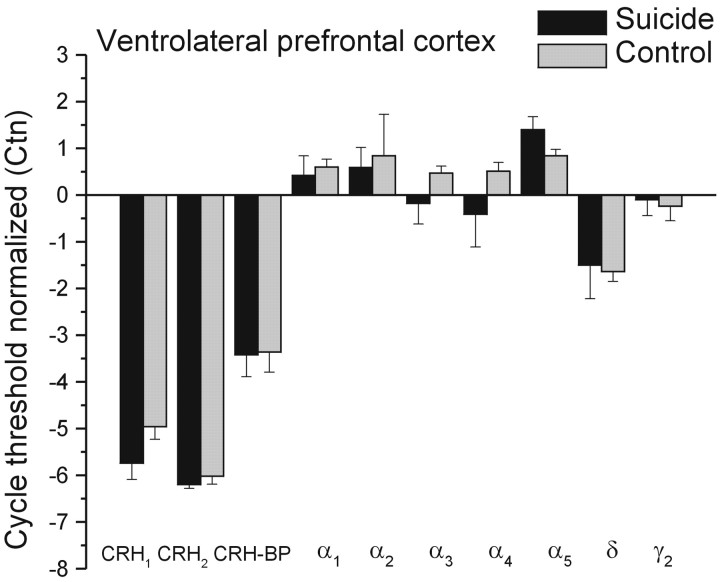
Within the VLPFC, differences between suicide and control samples were not evident with respect to the levels of CRH (mean ± SEM, 179.23 ± 35.66 vs 141.80 ± 9.07 pg/mg of protein) or mRNA expression of CRH receptors or GABAA subunits receptors (Fig. 3). Once again, the number of samples assayed was small, and the power to detect significant effects was limited. However, as seen in Figure 3 (relative to Figs. 1 and 2), the absolute difference between the suicide and comparison control brains, with respect to CRH receptor and particularly GABA subunit mRNA expression, was considerably smaller compared with that seen in the FPC. Indeed, in the case of the GABAA receptors (with the exception of the α5 subunit), the difference between suicide and control samples was generally less than one-third of a cycle. Thus, despite the small number of samples assayed, it is likely that the absence of a significant outcome was not simply related to the low power to detect such effects.
Figure 3.
Mean (±SEM) mRNA expression of CRH1, CRH2, and CRH-binding protein (CRH-BP), as well as mRNA expression of GABAA subunits in the ventrolateral prefrontal cortex of depressed suicides and controls. * p < 0.05 relative to control samples.
Cross-correlational analyses
To determine whether interrelationships existed among the CRH levels, CRH-binding protein, and CRH receptor expression, as well as between the expression of the different GABAA receptor subunits, cross-correlational analyses were conducted independently for each brain region among the depressed suicides and in the nondepressed control condition. With respect to the CRH and CRH receptor mRNA expression, a systematic profile of relationships was not apparent. Specifically, within the FPC, the correlational analyses among control participants indicated that CRH levels were not related to expression of the binding protein or CRH1 or CRH2 receptors (r = -0.21, 0.18, and 0.18, respectively). Among the suicide victims, CRH was unrelated to the binding protein (r = 0.31), but a moderate inverse correlation was evident in relation to CRH1 mRNA expression (r = -0.48; p < 0.10), and a direct relation to CRH2 receptor expression (r = 0.67; p < 0.01). Within the DMPFC, it was found that, among the control participants, CRH levels were not related to the CRH-binding protein (r = 0.04), CRH1 mRNA receptor expression (r = -0.29), or CRH2 receptor expression (r = -0.42). Among the depressed suicides, CRH was again found not to be related to the binding protein (r = 0.33), but was directly related to CRH1 mRNA expression (r = 0.69; p < 0.05) and inversely related to CRH2 receptor expression (r =-0.77; p < .01). In effect, among suicide victims, the profile of these relationships in the two regions, were opposite to one another.
Correlational analyses of the mRNA for the different GABA receptor subunits indicated that, in both the FPC and DMPFC of nondepressed individuals, significant interrelationships were frequent. As seen in Table 1, in the FPC of the control participants, 17 of 21 possible correlations were statistically significant (two-tailed tests). Specifically, each of the α receptor subunits were correlated with one another. The δ subunit was significantly correlated with the α2, α3, and α4 subunits, and the γ2 subunit was correlated significantly with the α1, α2, α4, and α5 subunits, and the correlation to the α3 subunit approached significance. Notable was the finding that the γ2 and δ subunits were unrelated. Importantly, when separate analyses were conducted among males and females, the profile of correlations was comparable with that evident using the complete sample.
Table 1.
Intercorrelations among GABA receptor subunits in the frontopolar cortex of suicide victims and controls
|
|
Controls |
Suicide victims |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
α1 |
α2 |
α3 |
α4 |
α5 |
δ |
γ |
α1 |
α2 |
α3 |
α4 |
α5 |
δ |
γ |
||||||||||||
| α1 | 0.85** | 0.79** | 0.85** | 0.70** | 0.52 | 0.87** | -0.14 | -0.18 | -0.26 | -0.07 | -0.44 | -0.09 | ||||||||||||||
| α2 | 0.80** | 0.95** | 0.68** | 0.72** | 0.71** | 0.96** | 0.92** | 0.15 | 0.51 | 0.44 | ||||||||||||||||
| α3 | 0.85** | 0.67** | 0.68** | 0.52 | 0.90** | 0.31 | 0.47 | 0.27 | ||||||||||||||||||
| α4 | 0.64** | 0.78** | 0.66* | 0.07 | 0.52 | 0.27 | ||||||||||||||||||||
| α5 | 0.54 | 0.61* | -0.26 | -0.05 | ||||||||||||||||||||||
| δ |
|
|
|
|
|
|
0.29 |
|
|
|
|
|
|
0.37 |
||||||||||||
*p<0.05, two-tailed test. **p<0.01, two-tailed test.
Within the DMPFC, the extent of the interrelationships, although extensive, were less marked than in the frontopolar cortex. Specifically, the α1 subunit was significantly related to the α2 and α4 subunits, the α2 subunit was also correlated to the α3, α4, and α5 subunits, and the α3, α4, and α5 subunits were all related to one another. Unlike the relationship between the δ receptors and the α subunits observed within the frontopolar cortex, within the FPC, the relationships of the α subunits to the δ and γ2 receptor subunits were uniformly nonsignificant.
In sharp contrast to the relationships seen in the tissue of nondepressed individuals, among suicide victims, only 3 of 21 correlations were significant in the FPC, and only 2 correlations were significant in the DMPFC (Table 2). In both regions, the α2 receptor subunit was related to the α3 and α4 subunits, and in the FPC, the latter two were related to each another. In effect, it seemed that the coordination between GABAA receptor subunits that ordinarily occurs within frontal regions in nondepressed individuals is not apparent in these regions of depressed suicides. Because four of the suicide victims died through drug overdose, the possibility, albeit slim, was considered that the inclusion of these tissue samples influenced the GABAA subunit of intercorrelations. When these four scores were removed from the analysis, the subunit mRNA levels were unaffected, and the intercorrelations between the subunits were remarkably similar to that seen with all of the brains included. The only exception was with regard to the correlations between the α1 and δ subunits within the FPC in which the correlations changed from -0.44 to -0.93. The meaningfulness of this change is uncertain, particularly because in controls this correlation was positive (0.52), and one of the few that did not reach statistical significance.
Table 2.
Intercorrelations among GABA receptor subunits in the DMPFC of suicide victims and controls
|
|
Controls |
Suicide victims |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
α1 |
α2 |
α3 |
α4 |
α5 |
δ |
γ2 |
α1 |
α2 |
α3 |
α4 |
α5 |
δ |
γ2 |
||||||||||||
| α1 | 0.71** | 0.57 | 0.64* | 0.34 | 0.49 | 0.44 | 0.50 | 0.44 | 0.60 | 0.50 | 0.25 | 0.00 | ||||||||||||||
| α2 | 0.81** | 0.85** | 0.81** | 0.50 | 0.17 | 0.80* | 0.87** | 0.16 | 0.33 | 0.27 | ||||||||||||||||
| α3 | 0.80** | 0.75** | 0.05 | 0.34 | 0.64 | -0.09 | 0.05 | 0.46 | ||||||||||||||||||
| α4 | 0.71** | 0.38 | 0.41 | 0.16 | 0.65 | 0.17 | ||||||||||||||||||||
| α5 | 0.19 | -0.19 | -0.16 | -0.35 | ||||||||||||||||||||||
| δ |
|
|
|
|
|
|
0.31 |
|
|
|
|
|
|
-0.33 |
||||||||||||
*p<0.05, two-tailed test. **p<0.01, two-tailed test.
Given the small number of lateral prefrontal cortex samples available, it was not felt that additional analyses of interrelationships would yield meaningful results, although it was clear that these relationships were as pronounced as in the other frontal areas examined (data not shown).
Discussion
Levels of CRH, CRH receptor, and GABAA subunit mRNA expression were examined in the granular frontopolar cortex (Brodmann 10), the dysgranular dorsomedial prefrontal area (Brodmann 9), and the ventrolateral prefrontal area (Brodmann 11). To a certain extent, all three areas are involved in cognitive processing as well as social behavior, emotionality, memory functions, and stress responses. Indeed, it was shown that morphological and neurochemical disturbances within frontal cortical regions accompany depressive illness (Drevets, 2000, 2001; Rajkowska, 2000). These disturbances included reductions not only of neurons and glial cells (Rajkowska, 2000), but also of cAMP binding sites and decreased protein kinase A activity (Dwivedi et al., 2002). In addition, depression was associated with a constellation of 5-HT neuronal changes, including decreased 5-HT transporter sites, increased 5-HT1A receptors (Arango et al., 1995; Mann et al., 2000), and increased 5HT2A receptors in prefrontal cortex (Zanardi et al., 2001; Pandey et al., 2002).
In addition, CRH hyperfunctioning within hypothalamic and extrahypothalamic sites (locus ceruleus, amygdala, hippocampus, nucleus accumbens, and prefrontal cortex) has been implicated in relatively severe (melancholic) depression or in which an abnormal dexamethasone response was evident (Reul and Holsboer, 2002). It had been reported that CRH binding sites were reduced in the frontal cortex of depressed suicides (Nemeroff et al., 1988), but other investigators did not observe such an outcome (Leake et al., 1990; Hucks et al., 1997). In the present investigation, both the CRH and GABAA systems within different aspects of the frontal cortex were affected among depressed suicides. CRH content itself was elevated in both the frontopolar cortex and dorsomedial prefrontal cortex, but not in the ventrolateral prefrontal cortex. Moreover, CRH1 receptor mRNA was reduced in the frontopolar cortex, and to a lesser extent in the dorsomedial prefrontal cortex, findings consistent with the view that CRH variations within these regions are related to depression (Nemeroff, 1996, 2002; Reul and Holsboer, 2002). Inasmuch as CRH1 desensitization may develop within hours after exposure to physiological concentrations of CRH (Pozzoli et al., 1996; Aguilera et al., 2001; Roseboom et al., 2001), the diminished CRH1 receptor expression in depressed suicides may have been secondary to a sustained increase of CRH activity (Nemeroff et al., 1988). Whatever the case, the finding that CRH1 but not CRH2 receptor mRNA was reduced, supports the contention that only the CRH1 subtype is aligned with mood disorders (Nemeroff, 1996, 2002; Dautzenberg and Hauger, 2002; Reul and Holsboer, 2002). Although animal studies concur with this position (Steckler and Holsboer, 1999), it still may be premature to dismiss a role for CRH2 receptors, particularly as such effects may be strain, sex, or situation specific (Bale et al., 2000).
Despite the pharmacological evidence consistent with a role for GABA in depression (Krystal et al., 2002; Brambilla et al., 2003), limited data are available concerning GABA changes in postmortem brain. Although Cheetham et al. (1988) reported elevated GABAA receptors, others reported that neither GABA levels nor GABAB binding was altered in depressed suicides (Cross et al., 1990; Arranz et al., 1992; Sundman et al., 1997). The present findings indicated that GABAA subunit mRNA expression, particularly with respect to the α1, α3, α4, and δ receptor subunits, was lower in depressed suicides than in controls, and that these effects were regionally specific, being evident in the frontopolar cortex, but absent in both the dorsomedial and ventrolateral prefrontal cortex.
Interestingly, a marked coordination was detected with respect to the mRNA expression of GABAA subunits in nondepressed individuals. Specifically, subunit transcript amounts were highly intercorrelated in the frontopolar cortex and to a somewhat lesser extent in the dorsomedial prefrontal cortex. One notable difference between the two regions was that the δ and γ2 receptors were only related to the α receptor subunits in the frontopolar cortex. Surprisingly, these relationships were mostly absent in the depressed suicide victims. These data imply that the overall regulation of expression of many of these subunits may be coordinated at a transcriptional level and this coordination is disturbed in suicide victims. Notable in this regard is that this dysregulation also occurred in an area (dorsomedial prefrontal cortex) in which, on average, the overall expression of the subunits was not different between groups. The reason for the differences between subunit coordination in depressed suicide and control brains is not clear. The simplest explanation is that, in the suicide brain, the genes are dysregulated and transcriptional control has been lost. Alternatively, mechanisms regulating RNA processing (e.g., poly-adenylation) may be altered, hence affecting mRNA stability. The latter possibility, however, is unlikely, because some of the transcripts were unaltered, suggesting a specificity of effect.
Studies showing coordinated expression of GABAA receptors subunits are sparse. However, it has been reported that, within dentate gyrus granule neurons, δ, α4, and β2 mRNA expression was highly correlated, as was that of α3 and β2 (Brooks-Kayal et al., 2001). Interestingly, it was noted that, because the genes for the α4, β2, and δ subunits appeared on different chromosomes, the subunit coordination could not be attributed to factors related to shared chromosomal localization (Brooks-Kayal et al., 2001). Although the present findings provide prima facie evidence linking interrelationships of the GABAA subunit mRNA expression with suicide, it still needs to be determined whether these interrelationships are present in other brain regions, and whether such relationships would be absent in those regions that are typically thought not to be involved in depressive illness or suicidality.
The implications of the disturbed subunit expression are unclear. Assuming that a concomitant decrease in protein expression occurs, these findings suggest the presence of a decrease in inhibitory tone. Because the tissue samples were heterogeneous, containing all of the cell layers and types (both inhibitory and excitatory), it is not clear in which cell population the subunit expression was altered. Inasmuch as CRH levels were increased, it seems likely that the interneuronal population may have been hyperexcited, likely reflecting the loss of inhibition on the interneurons. Alternatively, this hyperactivity may reflect an enhancement of recurrent excitation from increased firing of the principal neurons.
The consequence of an imbalance of inhibition in frontal cortical regions (as indicated by the loss of coordinated expression) is speculative. It has been established that variations of α subunit expression is associated with altered timing of inhibition, which is an important regulator of neural network behavior (Buzsaki, 2001). As such, one would predict altered firing patterns in the suicide brain. However, it is not possible to predict precisely how profound these altered timing patterns might be. As indicated by Buzsaki (2001), if the timing of inhibition is altered in the interneurons, then changes of network behavior may be profound, but if the changes are restricted to the principal cells, then less dramatic changes would be manifested.
It is premature to offer strong positions concerning the processes subserving the interrelationships among CRH, CRH receptors, and the GABA receptor subunits in depressed suicides. Among other things, it remains to be determined whether the CRH and GABAA receptor subunit changes, or the disturbed coordination between these GABAA receptor subunits, contribute to depression or are secondary to the illness or the stressors associated with it. Furthermore, the changes within these systems may be independent of each other, with one system affecting a particular attribute of the wide range of behavioral symptoms that comprise the depressive syndrome, and the second system being associated with other characteristics of depressed suicides. It is equally possible, however, that the CRH and GABA systems are related to each other and together impact the symptom profile of the depressed suicide. In this respect, it was reported that GABAergic agents may influence limbic CRH mRNA expression (Cullinan, 2000; Skelton et al., 2000; Stout et al., 2001; Gilmor et al., 2003), and hence may affect the stressor-provoked CRH response (Cullinan and Wolfe, 2000). Thus, just as CRH may influence monoaminergic (both NE and 5-HT) neuronal functioning, which could potentially influence depression (Ruggiero et al., 1999), CRH-GABA interrelationships may exist that influence affective states.
The current findings suggest several novel avenues of research, but our conclusions are not without limitations. The number of subjects in each condition was limited, and it is premature to discount sex as a factor pertinent to the peptide or receptor differences detected. Although the tight integration of the GABAA subunits was apparent in both male and female nondepressed samples, but absent in the suicide condition that comprised primarily males, it cannot be excluded that the dysregulation is sex dependent. Furthermore, suicide may be a unique entity, reflecting the culmination of several complex processes (i.e., not simply depression, but also impulsivity, disinhibition, anxiety, executive functioning) (van Heeringen, 2001), each of which may differ across individuals, and may not reflect those that govern major depression itself. Finally, follow-up studies are necessary to demonstrate the cellular distribution of the mRNA changes, and to what degree these changes have an impact on the protein distribution. These caveats notwithstanding, the present report is the first to show the integration of GABAA subunit mRNAs in frontal brain regions, and the first to demonstrate that dysregulation occurs in association with a psychopathological state.
Footnotes
This work was supported by a grant from the Canadian Institutes of Health Research (H.A., M.P., Z.M.) and by Hungarian National Research Grant (Hungarian National Science Foundation) 034496 (M.P.). H.A. holds a Canada Research Chair in Neuroscience and is an Ontario Mental Health Senior Research Fellow.
Correspondence should be addressed to Dr. Hymie Anisman, Institute of Neuroscience, Carleton University, Ottawa, Ontario K1S 5B6, Canada. E-mail: hanisman@ccs.carleton.ca.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241478-08$15.00/0
References
- Aguilera G, Rabadan-Diehl C, Nikodemova M (2001) Regulation of pituitary corticotropin-releasing hormone receptors. Peptides 22: 769-774. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ (1995) Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 688: 1038-1047. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160: 1-12. [DOI] [PubMed] [Google Scholar]
- Arranz B, Cowburn R, Eriksson A, Vestling M, Marcusson J (1992) γ-Aminobutyric acid-B (GABAB) binding sites in postmortem suicide brains. Neuropsychobiology 26: 33-36. [DOI] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA (2003) Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry 8: 324-332. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino AB, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF (2000) Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410-414. [DOI] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G (2003) Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 28: 1328-1335. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC (2003) GABAergic dysfunction in mood disorders. Mol Psychiatry 8: 721-737. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA (2001) γ-Aminobutyric acidA receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem 77: 1266-1278. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2001) Hippocampal GABAergic interneurons: a physiological perspective. Neurochem Res 26: 899-905. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Parker SJ, Horton RW (1988) Brain GABAA/benzodiazepine binding sites and glutamic acid decarboxylase activity in depressed suicide victims. Brain Res 460: 114-123. [DOI] [PubMed] [Google Scholar]
- Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, Becker AJ (2001) Activity-induced expression of common reference genes in individual CNS neurons. Lab Invest 81: 913-916. [DOI] [PubMed] [Google Scholar]
- Cross JA, Cheetham SC, Crompton MR, Katona CL, Horton RW (1990) Brain GABAB binding sites in depressed suicide victims. Psychiatry Res 26: 119-129. [DOI] [PubMed] [Google Scholar]
- Cullinan WE (2000) GABAA receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol 419: 344-351. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Wolfe TJ (2000) Chronic stress regulates levels of mRNA transcripts encoding β subunits of the GABAA receptor in the rat stress axis. Brain Res 887: 118-124. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23: 71-77. [DOI] [PubMed] [Google Scholar]
- Drevets WC (2000) Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813-8129. [DOI] [PubMed] [Google Scholar]
- Drevets WC (2001) Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 11: 240-249. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2002) [3H]cAMP binding sites and protein kinase A activity in the prefrontal cortex of suicide victims. Am J Psychiatry 159: 66-73. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Skelton KH, Nemeroff CB, Owens MJ (2003) The effects of chronic treatment with the mood stabilizers valproic acid and lithium on corticotropin-releasing factor neuronal systems. J Pharmacol Exp Ther 305: 434-439. [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F (1998) Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 8: 71-79. [PubMed] [Google Scholar]
- Holsboer F (2003) Corticotropin-releasing hormone modulators and depression. Curr Opin Investig Drugs 4: 46-50. [PubMed] [Google Scholar]
- Honig A, Bartlett JR, Bouras N, Bridges PK (1988) Amino acid levels in depression: a preliminary investigation. J Psychiatr Res 22: 159-164. [DOI] [PubMed] [Google Scholar]
- Hucks D, Lowther S, Crompton MR, Katona CL, Horton RW (1997) Corticotropin-releasing factor binding sites in cortex of depressed suicides. Psychopharmacology 134: 174-178. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M (1999) Neural systems for behavioral activation and reward. Curr Opin Neurobiol 9: 223-227. [DOI] [PubMed] [Google Scholar]
- Kling MA, Roy A, Doran AR, Calabrese JR, Rubinow DR, Whitfield Jr HJ, May C, Post RM, Chrousos GP, Gold PW (1991) Cerebrospinal fluid immunoreactive corticotropin-releasing hormone and adrenocorticotropin secretion in Cushing's disease and major depression: potential clinical implications. J Clin Endocrinol Metab 72: 260-271. [DOI] [PubMed] [Google Scholar]
- Kling MA, Geracioti TD, Licinio J, Michelson D, Oldfield EH, Gold PW (1994) Effects of electroconvulsive therapy on the CRH-ACTH-cortisol system in melancholic depression: preliminary findings. Psychopharmacol Bull 30: 489-494. [PubMed] [Google Scholar]
- Korpi ER, Kleinman JE, Wyatt RJ (1988) GABA concentrations in forebrain areas of suicide victims. Biol Psychiatry 23: 109-114. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF (2002) Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 7: S71-S80. [DOI] [PubMed] [Google Scholar]
- Leake A, Perry EK, Perry RH, Fairbairn AF, Ferrier IN (1990) Cortical concentrations of corticotropin-releasing hormone and its receptor in Alzheimer type dementia and major depression. Biol Psychiatry 28: 603-608. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Zivkovic B, Scatton B, Morselli PL, Bartholini G (1989) The GABAergic hypothesis of depression. Prog Neuropsychopharmacol Biol Psychiatry 13: 341-351. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V (2000) A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 57: 729-738. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H (1998) Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 18: 4758-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ (1998) The role of corticotropin-releasing factor in depressive illness: a critical review. Neurosci Biobehav Rev 22: 635-651. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB (1996) The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1: 336-342. [PubMed] [Google Scholar]
- Nemeroff CB (2002) New directions in the development of antidepressants: the interface of neurobiology and psychiatry. Hum Psychopharmacol 17 [Suppl 1]: S13-S16. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ (2003) Treatment of mood disorders. Nat Neurosci 5: 1068-1070. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226: 1342-1344. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M (1988) Reduced corticotropin-releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45: 577-579. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, Fink M (1991) Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy: corticotropin-releasing factor, β-endorphin and somatostatin. Br J Psychiatry 158: 59-63. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ (2003) Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci 23: 7931-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, Roberts RC, Conley RR, Tamminga CA (2002) Higher expression of serotonin 5-HT2A receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry 159: 419-429. [DOI] [PubMed] [Google Scholar]
- Petty F (1995) GABA and mood disorders: a brief review and hypothesis. J Affect Disord 34: 275-281. [DOI] [PubMed] [Google Scholar]
- Pozzoli G, Bilezikjian LM, Perrin MH, Blount AL, Vale WW (1996) Corticotropin-releasing factor (CRF) and glucocorticoids modulate the expression of type 1 CRF receptor messenger ribonucleic acid in rat anterior pituitary cell cultures. Endocrinology 137: 65-71. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF (1994) Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60: 436-444. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF (1995) Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry 152: 1372-1376. [DOI] [PubMed] [Google Scholar]
- Rajkowska G (2000) Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res 126: 397-412. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, Holsboer F (2002) Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharamacol 2: 23-33. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Urben CM, Kalin NH (2001) Persistent corticotropin-releasing factor1 receptor desensitization and downregulation in the human neuroblastoma cell line IMR-32. Brain Res Mol Brain Res 92: 115-127. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Paul S, Doran A, Chrousos GP, Gold PW (1987) CSF corticotropin-releasing hormone in depressed patients and normal control subjects. Am J Psychiatry 144: 641-645. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V (1999) Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience 91: 1343-1354. [DOI] [PubMed] [Google Scholar]
- Sherif F, Marcusson J, Oreland L (1991) Brain γ-aminobutyrate transaminase and monoamine oxidase activities in suicide victims. Eur Arch Psychiatry Clin Neurosci 241: 139-144. [DOI] [PubMed] [Google Scholar]
- Shiah IS, Yatham LN (1998) GABA function in mood disorders: an update and critical review. Life Sci 63: 1289-1303. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ (2000) Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci 20: 1240-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Holsboer M (1999) Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry 46: 1480-1508. [DOI] [PubMed] [Google Scholar]
- Stout SC, Owens MJ, Lindsey KP, Knight DL, Nemeroff CB (2001) Effects of sodium valproate on corticotropin-releasing factor systems in rat brain. Neuropsychopharmacology 24: 624-631. [DOI] [PubMed] [Google Scholar]
- Sundman I, Allard P, Eriksson A, Marcusson J (1997) GABA uptake sites in frontal cortex from suicide victims and in aging. Neuropsychobiology 35: 11-15. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E (2001) Behavioral planning in the prefrontal cortex. Curr Opin Neurobiol 11: 164-170. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G, Malatynska E (2003) Central GABAergic systems and depressive illness. Neurochem Res 28: 965-976. [DOI] [PubMed] [Google Scholar]
- Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, Rivier J (1983) Assay of corticotropin-releasing factor. Methods Enzymol 103: 565-577. [DOI] [PubMed] [Google Scholar]
- van Heeringen C (2001) Suicide, serotonin, and the brain. Crisis 22: 66-70. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti Jr TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW (2000) Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 97: 325-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Baram TZ, Gerth A, Schultz L, Ribak CE (1998) Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Exp Brain Res 123: 334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi R, Artigas F, Moresco R, Colombo C, Messa C, Gobbo C, Smeraldi E, Fazio F (2001) Increased 5-hydroxytryptamine-2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol 21: 53-58. [DOI] [PubMed] [Google Scholar]